Ventromedial hypothalamic glucose sensing and glucose homeostasis vary throughout the estrous cycle
- PMID: 27666162
- PMCID: PMC5159237
- DOI: 10.1016/j.physbeh.2016.09.021
Ventromedial hypothalamic glucose sensing and glucose homeostasis vary throughout the estrous cycle
Abstract
Objective: 17β-Estradiol (17βE) regulates glucose homeostasis in part by centrally mediated mechanisms. In female rodents, the influence of the ovarian cycle on hypoglycemia counterregulation and glucose tolerance is unclear. We found previously that in prepubertal females, 17βE modulates glucose sensing in nonadapting glucose-inhibited (GI) and adapting GI (AdGI) neurons within the ventrolateral portion of the ventromedial nucleus (VL-VMN). Nonadapting GI neurons persistently decrease their activity as glucose increases while AdGI neurons transiently respond to a glucose increase. To begin to understand if endogenous fluctuations in estrogen levels across the estrous cycle impact hypothalamic glucose sensing and glucose homeostasis, we assessed whether hypoglycemia counterregulation and glucose tolerance differed across the phases of the estrous cycle. We hypothesized that the response to insulin-induced hypoglycemia (IIH) and/or glucose tolerance would vary throughout the estrous cycle according to changes in 17βE availability. Moreover, that these changes would correlate with estrous-dependent changes in the glucose sensitivity of VL-VMN glucose-sensing neurons (GSNs).
Methods: These hypotheses were tested in female mice by measuring the response to IIH, glucose tolerance and the glucose sensitivity of VL-VMN GSNs during each phase of the estrous cycle. Furthermore, a physiological brain concentration of 17βE seen during proestrus was acutely applied to brain slices isolated on the day of diestrous and the response to low glucose in VL-VMN GSNs was assayed.
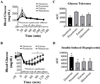
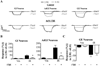
Results: The response to IIH was strongest during diestrous. The response of nonadapting GI and AdGI neurons to a glucose decrease from 2.5 to 0.5mM also peaked during diestrous; an effect which was blunted by the addition of 17βE. In contrast, the glucose sensitivity of the subpopulation of GSNs which are excited by glucose (GE) was not affected by estrous phase or exogenous 17βE application.
Conclusion: These data suggest that physiological fluctuations in circulating 17βE levels across the estrous cycle lead to changes in hypothalamic glucose sensing and the response to IIH.
Keywords: Estrogen; Glucose tolerance; Glucose-excited neurons; Glucose-inhibited neurons; Hypoglycemia counterregulation.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Estrogens modulate ventrolateral ventromedial hypothalamic glucose-inhibited neurons.Mol Metab. 2016 Aug 20;5(10):823-833. doi: 10.1016/j.molmet.2016.08.002. eCollection 2016 Oct. Mol Metab. 2016. PMID: 27688996 Free PMC article.
-
Changes in neuronal activity across the mouse ventromedial nucleus of the hypothalamus in response to low glucose: Evaluation using an extracellular multi-electrode array approach.J Neuroendocrinol. 2020 Mar;32(3):e12824. doi: 10.1111/jne.12824. Epub 2020 Feb 23. J Neuroendocrinol. 2020. PMID: 31880369 Free PMC article.
-
A Golgi study of the plasticity of dendritic spines in the hypothalamic ventromedial nucleus during the estrous cycle of female rats.Neuroscience. 2015 Jul 9;298:74-80. doi: 10.1016/j.neuroscience.2015.04.019. Epub 2015 Apr 16. Neuroscience. 2015. PMID: 25892700
-
Glucose sensing neurons in the ventromedial hypothalamus.Sensors (Basel). 2010;10(10):9002-25. doi: 10.3390/s101009002. Sensors (Basel). 2010. PMID: 22022208 Free PMC article. Review.
-
Hypothalamic nitric oxide in hypoglycemia detection and counterregulation: a two-edged sword.Antioxid Redox Signal. 2011 Feb 1;14(3):505-17. doi: 10.1089/ars.2010.3331. Epub 2010 Aug 17. Antioxid Redox Signal. 2011. PMID: 20518706 Free PMC article. Review.
Cited by
-
Bone morphogenetic protein 8B (BMP8B) increases the glucose sensitivity of ventromedial hypothalamus (VMH) glucose-inhibited (GI) neurons in female mice.MicroPubl Biol. 2025 Mar 18;2025:10.17912/micropub.biology.001496. doi: 10.17912/micropub.biology.001496. eCollection 2025. MicroPubl Biol. 2025. PMID: 40181907 Free PMC article.
-
Sex and gender differences in developmental programming of metabolism.Mol Metab. 2018 Sep;15:8-19. doi: 10.1016/j.molmet.2018.04.007. Epub 2018 Apr 30. Mol Metab. 2018. PMID: 29773464 Free PMC article. Review.
-
Glycerol not lactate is the major net carbon source for gluconeogenesis in mice during both short and prolonged fasting.Mol Metab. 2020 Jan;31:36-44. doi: 10.1016/j.molmet.2019.11.005. Epub 2019 Nov 9. Mol Metab. 2020. PMID: 31918920 Free PMC article.
-
Ventromedial Nucleus of the Hypothalamus Neurons Under the Magnifying Glass.Endocrinology. 2021 Oct 1;162(10):bqab141. doi: 10.1210/endocr/bqab141. Endocrinology. 2021. PMID: 34265067 Free PMC article. Review.
-
Emergent decision-making behaviour and rhythm generation in a computational model of the ventromedial nucleus of the hypothalamus.PLoS Comput Biol. 2019 Jun 3;15(6):e1007092. doi: 10.1371/journal.pcbi.1007092. eCollection 2019 Jun. PLoS Comput Biol. 2019. PMID: 31158265 Free PMC article.
References
-
- López M, Tena-Sempere M. Estrogens and the control of energy homeostasis: a brain perspective. Trends in Endocrinology & Metabolism. 2015;26:411–421. - PubMed
-
- Gonzalez-Ortiz M, Martinez-Abundis E, Lifshitz A. Insulin sensitivity and sex steroid hormone levels during the menstrual cycle in healthy women with non-insulin-dependent diabetic parents. Gynecol Obstet Invest. 1998;46:187–190. - PubMed
-
- Escalante Pulido JM, Alpizar Salazar M. Changes in insulin sensitivity, secretion and glucose effectiveness during menstrual cycle. Arch Med Res. 1999;30:19–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

