Biallelic Mutations in TBCD, Encoding the Tubulin Folding Cofactor D, Perturb Microtubule Dynamics and Cause Early-Onset Encephalopathy
- PMID: 27666370
- PMCID: PMC5065658
- DOI: 10.1016/j.ajhg.2016.08.003
Biallelic Mutations in TBCD, Encoding the Tubulin Folding Cofactor D, Perturb Microtubule Dynamics and Cause Early-Onset Encephalopathy
Abstract
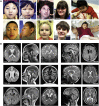
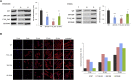
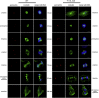
Microtubules are dynamic cytoskeletal elements coordinating and supporting a variety of neuronal processes, including cell division, migration, polarity, intracellular trafficking, and signal transduction. Mutations in genes encoding tubulins and microtubule-associated proteins are known to cause neurodevelopmental and neurodegenerative disorders. Growing evidence suggests that altered microtubule dynamics may also underlie or contribute to neurodevelopmental disorders and neurodegeneration. We report that biallelic mutations in TBCD, encoding one of the five co-chaperones required for assembly and disassembly of the αβ-tubulin heterodimer, the structural unit of microtubules, cause a disease with neurodevelopmental and neurodegenerative features characterized by early-onset cortical atrophy, secondary hypomyelination, microcephaly, thin corpus callosum, developmental delay, intellectual disability, seizures, optic atrophy, and spastic quadriplegia. Molecular dynamics simulations predicted long-range and/or local structural perturbations associated with the disease-causing mutations. Biochemical analyses documented variably reduced levels of TBCD, indicating relative instability of mutant proteins, and defective β-tubulin binding in a subset of the tested mutants. Reduced or defective TBCD function resulted in decreased soluble α/β-tubulin levels and accelerated microtubule polymerization in fibroblasts from affected subjects, demonstrating an overall shift toward a more rapidly growing and stable microtubule population. These cells displayed an aberrant mitotic spindle with disorganized, tangle-shaped microtubules and reduced aster formation, which however did not alter appreciably the rate of cell proliferation. Our findings establish that defective TBCD function underlies a recognizable encephalopathy and drives accelerated microtubule polymerization and enhanced microtubule stability, underscoring an additional cause of altered microtubule dynamics with impact on neuronal function and survival in the developing brain.
Copyright © 2016 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Biallelic TBCD Mutations Cause Early-Onset Neurodegenerative Encephalopathy.Am J Hum Genet. 2016 Oct 6;99(4):950-961. doi: 10.1016/j.ajhg.2016.08.005. Epub 2016 Sep 22. Am J Hum Genet. 2016. PMID: 27666374 Free PMC article.
-
TBCE Mutations Cause Early-Onset Progressive Encephalopathy with Distal Spinal Muscular Atrophy.Am J Hum Genet. 2016 Oct 6;99(4):974-983. doi: 10.1016/j.ajhg.2016.08.006. Epub 2016 Sep 22. Am J Hum Genet. 2016. PMID: 27666369 Free PMC article.
-
Microcephaly, intractable seizures and developmental delay caused by biallelic variants in TBCD: further delineation of a new chaperone-mediated tubulinopathy.Clin Genet. 2017 May;91(5):725-738. doi: 10.1111/cge.12914. Epub 2016 Dec 16. Clin Genet. 2017. PMID: 27807845
-
Developmental Regression and Epilepsy of Infancy with Migrating Focal Seizures Caused by TBCD Mutation: A Case Report and Review of the Literature.Neuropediatrics. 2020 Feb;51(1):68-71. doi: 10.1055/s-0039-1698423. Epub 2019 Sep 30. Neuropediatrics. 2020. PMID: 31569255 Review.
-
Tubulin mutations in neurodevelopmental disorders as a tool to decipher microtubule function.FEBS Lett. 2020 Nov;594(21):3409-3438. doi: 10.1002/1873-3468.13958. Epub 2020 Nov 3. FEBS Lett. 2020. PMID: 33064843 Review.
Cited by
-
HIPK2-T566 autophosphorylation diversely contributes to UV- and doxorubicin-induced HIPK2 activation.Oncotarget. 2017 Mar 7;8(10):16744-16754. doi: 10.18632/oncotarget.14421. Oncotarget. 2017. PMID: 28060750 Free PMC article.
-
Infant mortality: the contribution of genetic disorders.J Perinatol. 2019 Dec;39(12):1611-1619. doi: 10.1038/s41372-019-0451-5. Epub 2019 Aug 8. J Perinatol. 2019. PMID: 31395954 Free PMC article.
-
Biallelic SQSTM1 mutations in early-onset, variably progressive neurodegeneration.Neurology. 2018 Jul 24;91(4):e319-e330. doi: 10.1212/WNL.0000000000005869. Epub 2018 Jun 29. Neurology. 2018. PMID: 29959261 Free PMC article.
-
A Trimer Consisting of the Tubulin-specific Chaperone D (TBCD), Regulatory GTPase ARL2, and β-Tubulin Is Required for Maintaining the Microtubule Network.J Biol Chem. 2017 Mar 10;292(10):4336-4349. doi: 10.1074/jbc.M116.770909. Epub 2017 Jan 26. J Biol Chem. 2017. PMID: 28126905 Free PMC article.
-
Single-cell RNA-seq variant analysis for exploration of genetic heterogeneity in cancer.Sci Rep. 2019 Jul 2;9(1):9524. doi: 10.1038/s41598-019-45934-1. Sci Rep. 2019. PMID: 31267007 Free PMC article.
References
-
- Kapitein L.C., Hoogenraad C.C. Building the neuronal microtubule cytoskeleton. Neuron. 2015;87:492–506. - PubMed
-
- Jaworski J., Kapitein L.C., Gouveia S.M., Dortland B.R., Wulf P.S., Grigoriev I., Camera P., Spangler S.A., Di Stefano P., Demmers J. Dynamic microtubules regulate dendritic spine morphology and synaptic plasticity. Neuron. 2009;61:85–100. - PubMed
-
- Breuss M., Keays D.A. Microtubules and neurodevelopmental disease: the movers and the makers. Adv. Exp. Med. Biol. 2014;800:75–96. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

