Role of phosphatase of regenerating liver 1 (PRL1) in spermatogenesis
- PMID: 27666520
- PMCID: PMC5035919
- DOI: 10.1038/srep34211
Role of phosphatase of regenerating liver 1 (PRL1) in spermatogenesis
Abstract
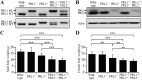
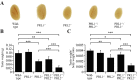
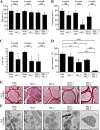
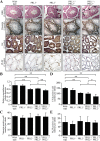
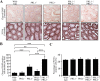
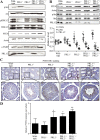
The PRL phosphatases are oncogenic when overexpressed but their in vivo biological function is less well understood. Previous gene deletion study revealed a role for PRL2 in spermatogenesis. We report here the first knockout mice lacking PRL1, the most related homolog of PRL2. We found that loss of PRL1 does not affect spermatogenesis and reproductive ability of male mice, likely due to functional compensation by the relatively higher expression of PRL2 in the testes. However, PRL1-/-/PRL2+/- male mice show testicular atrophy phenotype similar to PRL2-/- mice. More strikingly, deletion of one PRL1 allele in PRL2-/- male mice causes complete infertility. Mechanistically, the total level of PRL1 and PRL2 is negatively correlated with the PTEN protein level in the testis and PRL1+/-/PRL2-/- mice have the highest level of PTEN, leading to attenuated Akt activation and increased germ cell apoptosis, effectively halting spermatozoa production. These results provide the first evidence that in addition to PRL2, PRL1 is also required for spermatogenesis by downregulating PTEN and promoting Akt signaling. The ability of the PRLs to suppress PTEN expression underscores the biochemical basis for their oncogenic potential.
Figures
Similar articles
-
Targeting PRL phosphatases in hematological malignancies.Expert Opin Ther Targets. 2024 Apr;28(4):259-271. doi: 10.1080/14728222.2024.2344695. Epub 2024 Apr 26. Expert Opin Ther Targets. 2024. PMID: 38653737 Free PMC article. Review.
-
Phosphatase of regenerating liver 2 (PRL2) deficiency impairs Kit signaling and spermatogenesis.J Biol Chem. 2014 Feb 7;289(6):3799-810. doi: 10.1074/jbc.M113.512079. Epub 2013 Dec 26. J Biol Chem. 2014. PMID: 24371141 Free PMC article.
-
Phosphatase of regenerating liver 2 (PRL2) is essential for placental development by down-regulating PTEN (Phosphatase and Tensin Homologue Deleted on Chromosome 10) and activating Akt protein.J Biol Chem. 2012 Sep 14;287(38):32172-9. doi: 10.1074/jbc.M112.393462. Epub 2012 Jul 12. J Biol Chem. 2012. PMID: 22791713 Free PMC article.
-
Mechanism of PRL2 phosphatase-mediated PTEN degradation and tumorigenesis.Proc Natl Acad Sci U S A. 2020 Aug 25;117(34):20538-20548. doi: 10.1073/pnas.2002964117. Epub 2020 Aug 11. Proc Natl Acad Sci U S A. 2020. PMID: 32788364 Free PMC article.
-
Phosphatase of regenerating liver in hematopoietic stem cells and hematological malignancies.Cell Cycle. 2014;13(18):2827-35. doi: 10.4161/15384101.2014.954448. Cell Cycle. 2014. PMID: 25486470 Free PMC article. Review.
Cited by
-
Regulatory Mechanisms and Novel Therapeutic Targeting Strategies for Protein Tyrosine Phosphatases.Chem Rev. 2018 Feb 14;118(3):1069-1091. doi: 10.1021/acs.chemrev.7b00105. Epub 2017 May 25. Chem Rev. 2018. PMID: 28541680 Free PMC article. Review.
-
Targeting PRL phosphatases in hematological malignancies.Expert Opin Ther Targets. 2024 Apr;28(4):259-271. doi: 10.1080/14728222.2024.2344695. Epub 2024 Apr 26. Expert Opin Ther Targets. 2024. PMID: 38653737 Free PMC article. Review.
-
A Novel Neuroprotective Role of Phosphatase of Regenerating Liver-1 against CO2 Stimulation in Drosophila.iScience. 2019 Sep 27;19:291-302. doi: 10.1016/j.isci.2019.07.026. Epub 2019 Jul 22. iScience. 2019. PMID: 31404830 Free PMC article.
-
PRL2 links magnesium flux and sex-dependent circadian metabolic rhythms.JCI Insight. 2017 Jul 6;2(13):e91722. doi: 10.1172/jci.insight.91722. eCollection 2017 Jul 6. JCI Insight. 2017. PMID: 28679948 Free PMC article.
-
Effect of Pharmacological Inhibition of the Catalytic Activity of Phosphatases of Regenerating Liver in Early T Cell Receptor Signaling Dynamics and IL-2 Production.Int J Mol Sci. 2020 Apr 5;21(7):2530. doi: 10.3390/ijms21072530. Int J Mol Sci. 2020. PMID: 32260565 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

