[Clinical Assessment of Chemosensitivity Test in Xeno-free Culture of Autologous Malignant Effusion Cells from Patients with Advanced Lung Adenocarcinoma]
- PMID: 27666547
- PMCID: PMC5972961
- DOI: 10.3779/j.issn.1009-3419.2016.09.04
[Clinical Assessment of Chemosensitivity Test in Xeno-free Culture of Autologous Malignant Effusion Cells from Patients with Advanced Lung Adenocarcinoma]
Abstract
Background: A great individual differences to chemotherapeutic effects existed in the patient with advanced lung cancer. How to choose the optimum regimens to achieve the individuation and maximum effect of chemotherapy for lung cancer is worth exploring. The study was designed to examine the effect of ex vitro chemo-sensitivity assay in xeno-free culture of autologous malignant effusion cells from patients with advanced lung adenocarcinoma.
Methods: The 50 treatment-naive patients with lung adenocarcinoma complicated with malignant pleural or pericardial effusions were enrolled. Effusions of all cases had been controlled by closed drainage and 300 mL-500 mL of which were retained under sterile condition from 25 cases (Chemo-sensitivity group). Primary malignant effusion cells were isolated from autologous effusions of the patients. Then, xeno-free culture (average 11 days) were intervened with 8 chemotherapeutic drugs commonly used in clinical practice and were determined by CCK-8 assay. Optimum regimens were selected for chemotherapy based on the results of chemosensitivity test. As a contrast, chemotherapy regimens for the other 25 patients (Control group) were on the basis of physician's clinical experience.
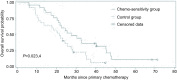
Results: After four cycles of chemotherapy, in Chemo-sensitivity group, 17 (68.0%) cases were determined for partial response (PR), 5 (20.0%) cases for stable disease (SD), and the objective response rate (ORR) was 68.0%, the disease control rate (DCR) was 88.0%. Meanwhile, in Control group, 9 (36.0%) cases were determined for PR, 7 (28.0%) cases for SD, and, the ORR was 36.0%, the DCR was 64.0%. There were significant differences between the two groups in ORR and DCR (P<0.05). To the end of follow-up, there were 21 cases of death in Chemo-sensitivity group as well as 22 cases in Control group. The mean progression-free survival (PFS) in Chemo-sensitivity group and Control group respectively were 10.0 months and 5.8 months, and the mean overall survival (OS) in the two groups were 30.2 months and 21.2 months respectively. There were also significant differences between the two groups in PFS and OS (P<0.05). Furthermore, the adverse reactions in both groups were mild and controllable.
Conclusions: Xeno-free culture of autologous malignant effusion cells from patients with advanced lung adenocarcinoma and ex vitro chemo-sensitivity assay are beneficial to the rational choices of chemotherapeutic agents used in patients with lung adenocarcinoma complicated with malignant effusions, which is a worthy trial in personalized cell culture for individualized cancer therapy and further studies.
背景与目的 晚期肺癌的化疗效果存在极大的个体差异,如何选择最佳化疗方案,实现肺癌化疗的个体化、效果最大化是值得探索的课题。本研究旨在检验自体积液中进行癌细胞培养及药敏试验对于合并胸腔或心包积液的肺腺癌患者优化化疗方案的临床应用价值。方法 收集50例并发恶性胸腔和/或心包积液的肺腺癌初治患者,经闭式引流控制积液。其中25例(药敏组)于无菌条件下留取积液300 mL-500 mL,肿瘤细胞通过自体积液 (xeno-free)平均11天的细胞培养而获得,继而针对8种临床常用化疗药物行药敏试验,通过CCK-8进行敏感性检测,根据试验结果选择最优化疗方案进行全身化疗;另25例(对照组)进行经验性化疗。结果 4个周期化疗后,药敏组部分缓解(partial response, PR)17例(68.0%)、稳定(stable disease, SD)5例(20.0%),客观缓解率(objective response rate, ORR)68.0%,疾病控制率(disease control rate, DCR)88.0%;对照组PR 9例(36.0%)、SD 7例(28.0%),ORR为36.0%,DCR为64.0%。比较两组ORR和DCR,差异均有统计学意义(P<0.05)。至随访截止,药敏组死亡21例,对照组死亡22例。药敏组无进展生存期(progression-free survival, PFS)平均10.0个月,总生存期(overall survival, OS)平均30.2个月;对照组PFS平均5.8个月,OS平均21.2个月。比较两组PFS和OS,差异均有统计学意义(P<0.05)。两组毒副反应轻微、可控。 结论 自体积液癌细胞培养及药敏试验有利于晚期肺腺癌患者化疗方案的合理选择。.
Figures

References
-
- Wrona A, Jassem J. The new TNM classification in lung cancer. http://www.ncbi.nlm.nih.gov/pubmed/21077033/ Pneumonol Alergol Pol. 2010;78(6):407–417. - PubMed
-
- Yang SF, Su JZ, Cao J, et al. Establishment of a novel Chinese human lung adenocarcinoma cell line CPA-Yang1 which produces highly bone metastases in immunodeficient mice. http://www.lungca.org/index.php?journal=01&page=article&op=view&path%5B%.... Zhongguo Fei Ai Za Zhi. 2009;12(7):753–759. - PubMed
- 杨 顺芳, 苏 建中, 曹 杰, et al. 中国人肺腺癌细胞系CPA-Yang1的建立及其生物学特性. http://www.lungca.org/index.php?journal=01&page=article&op=view&path%5B%... 中国肺癌杂志. 2009;12(7):753–759. - PubMed
-
- Yang SF, Su JZ, Shi MP, et al. Establishment and characterization of a novel Chinese human lung adenocarcinoma cell line CPA-Yang2 in immunodeficient mice. http://www.lungca.org/index.php?journal=01&page=article&op=view&path%5B%.... Zhongguo Fei Ai Za Zhi. 2009;12(10):1055–1060. doi: 10.3779/j.issn.1009-3419.2009.10.01. - DOI - PubMed
- 杨 顺芳, 苏 建中, 时 梅萍, et al. 中国人肺腺癌细胞系CPA-Yang2的建立及生物学特性. http://www.lungca.org/index.php?journal=01&page=article&op=view&path%5B%... 中国肺癌杂志. 2009;12(10):1055–1060. doi: 10.3779/j.issn.1009-3419.2009.10.01. - DOI - PubMed
-
- Yang SF, Shi MP, Cao J, et al. Establishment of a novel Chinese human lung adenocarcinoma cell line CPA-Yang3 and its real bone metastasis clone CPA-Yang3BM in immunodeficient mice. http://www.lungca.org/index.php?journal=01&page=article&op=view&path%5B%.... Zhongguo Fei Ai Za Zhi. 2011;14(2):79–85. - PMC - PubMed
- 杨 顺芳, 时 梅萍, 曹 杰, et al. 中国人肺腺癌细胞系CPA-Yang3及其骨转移细胞的建立. http://www.lungca.org/index.php?journal=01&page=article&op=view&path%5B%... 中国肺癌杂志. 2011;14(2):79–85. - PubMed
-
- Usta SN, Scharer CD, Xu J, et al. Chemically defined serum-free and xeno-free media for multiple cell lineages. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4205861/ Ann Transl Med. 2014;2(10):97–105. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

