Enantioselective Pd-Catalyzed Allylic Alkylation Reactions of Dihydropyrido[1,2-a]indolone Substrates: Efficient Syntheses of (-)-Goniomitine, (+)-Aspidospermidine, and (-)-Quebrachamine
- PMID: 27666731
- PMCID: PMC5207349
- DOI: 10.1002/anie.201608138
Enantioselective Pd-Catalyzed Allylic Alkylation Reactions of Dihydropyrido[1,2-a]indolone Substrates: Efficient Syntheses of (-)-Goniomitine, (+)-Aspidospermidine, and (-)-Quebrachamine
Abstract
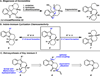
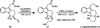
The successful application of dihydropyrido[1,2-a]indolone (DHPI) substrates in Pd-catalyzed asymmetric allylic alkylation chemistry facilitates rapid access to multiple alkaloid frameworks in an enantioselective fashion. Strategic bromination at the indole C3 position greatly improved the allylic alkylation chemistry and enabled a highly efficient Negishi cross-coupling downstream. The first catalytic enantioselective total synthesis of (-)-goniomitine, along with divergent formal syntheses of (+)-aspidospermidine and (-)-quebrachamine, are reported herein.
Keywords: alkaloids; allylic alkylation; asymmetric catalysis; quaternary centers; total synthesis.
© 2016 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
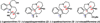
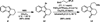


References
-
-
For reviews, see: Saxton JE. Alkaloids. 1998;51:1–197. O’Connor SE. J. J. Maresh, Nat. Prod. Rep. 2006;23:532–547.
-
-
-
For initial isolation of goniomitine (1) and proposed biosynthesis from vincadifformine (4), see: Randriambola L, Quirion J-C, Kan-Fan C, Husson H-P. Tetrahedron Lett. 1987;28:2123–2126.
-
-
- Biemann K, Friedmann-Spiteller M, Spiteller G. Tetrahedron Lett. 1961;2:485–492.
- Hesse O. Ber. Dtsch. Chem. Ges. 1880;13:2308–2309.
- Djerassi C, Budzikiewicz H, Wilson JM, Gosset J, Le Men J, Janot M-M. Tetrahedron Lett. 1962;3:235–239.
-
-
For a uniform numbering system of monoterpene indole alkaloids, see: Le Men J, Taylor WI. Experientia. 1965;21:508–510.
-
-
-
For a biomimetic semisynthesis of goniomitine (1) from vincadifformine (4), see: Lewin G, Bernadat G, Aubert G, Cresteil T. Tetrahedron. 2013;69:1622–1627.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

