How Azobenzene Photoswitches Restore Visual Responses to the Blind Retina
- PMID: 27667006
- PMCID: PMC5079435
- DOI: 10.1016/j.neuron.2016.08.038
How Azobenzene Photoswitches Restore Visual Responses to the Blind Retina
Abstract
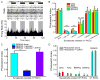
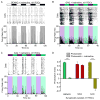
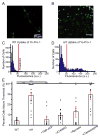
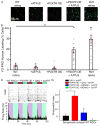
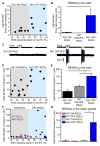
Azobenzene photoswitches confer light sensitivity onto retinal ganglion cells (RGCs) in blind mice, making these compounds promising candidates as vision-restoring drugs in humans with degenerative blindness. Remarkably, photosensitization manifests only in animals with photoreceptor degeneration and is absent from those with intact rods and cones. Here we show that P2X receptors mediate the entry of photoswitches into RGCs, where they associate with voltage-gated ion channels, enabling light to control action-potential firing. All charged photoswitch compounds require permeation through P2X receptors, whose gene expression is upregulated in the blind retina. Photoswitches and membrane-impermeant fluorescent dyes likewise penetrate through P2X receptors to label a subset of RGCs in the degenerated retina. Electrophysiological recordings and mapping of fluorescently labeled RGC dendritic projections together indicate that photosensitization is highly selective for OFF-RGCs. Hence, P2X receptors are a natural conduit allowing cell-type-selective and degeneration-specific delivery of photoswitches to restore visual function in blinding disease.
Keywords: P2X receptor; azobenzene; blindness; ion channel; photoswitch; retina; retinal degeneration; retinal ganglion cell; retinitis pigmentosa; vision.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures

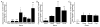

References
-
- Barboni MTS, Nagy BV, de Araújo Moura AL, Damico FM, da Costa MF, Kremers J, Ventura DF. ON and OFF Electroretinography and Contrast Sensitivity in Duchenne Muscular Dystrophy. Investigative ophthalmology & visual science. 2013;54:3195–3204. - PubMed
-
- Bemme S, Weick M, Gollisch T. Pharmacological blockade of HCN channels changes evoked and spontaneous activity of retinal ganglion cells. Investigative ophthalmology & visual science. 2014;55:2385–2385.
-
- Brandle U, Guenther E, Irrle C, Wheeler-Schilling TH. Gene expression of the P2X receptors in the rat retina. Brain research Molecular brain research. 1998;59:269–272. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

