Gamma-Rhythmic Gain Modulation
- PMID: 27667008
- PMCID: PMC5053905
- DOI: 10.1016/j.neuron.2016.09.003
Gamma-Rhythmic Gain Modulation
Abstract
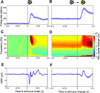
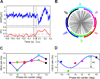
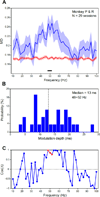
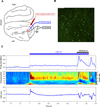
Cognition requires the dynamic modulation of effective connectivity, i.e., the modulation of the postsynaptic neuronal response to a given input. If postsynaptic neurons are rhythmically active, this might entail rhythmic gain modulation, such that inputs synchronized to phases of high gain benefit from enhanced effective connectivity. We show that visually induced gamma-band activity in awake macaque area V4 rhythmically modulates responses to unpredictable stimulus events. This modulation exceeded a simple additive superposition of a constant response onto ongoing gamma-rhythmic firing, demonstrating the modulation of multiplicative gain. Gamma phases leading to strongest neuronal responses also led to shortest behavioral reaction times, suggesting functional relevance of the effect. Furthermore, we find that constant optogenetic stimulation of anesthetized cat area 21a produces gamma-band activity entailing a similar gain modulation. As the gamma rhythm in area 21a did not spread backward to area 17, this suggests that postsynaptic gamma is sufficient for gain modulation.
Keywords: Channelrhodopsin; attention; communication-through-coherence (CTC); effective connectivity; gain; gamma; oscillation; rhythm; synchronization; visual cortex.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


References
-
- Azouz R, Gray CM. Adaptive coincidence detection and dynamic gain control in visual cortical neurons in vivo. Neuron. 2003;37:513–523. - PubMed
-
- Bastos AM, Vezoli J, Bosman CA, Schoffelen JM, Oostenveld R, Dowdall JR, De Weerd P, Kennedy H, Fries P. Visual areas exert feedforward and feedback influences through distinct frequency channels. Neuron. 2015a;85:390–401. - PubMed
-
- Bastos AM, Vezoli J, Fries P. Communication through coherence with interareal delays. Current opinion in neurobiology. 2015b;31:173–180. - PubMed
-
- Berens P. CircStat: A MATLAB Toolbox for Circular Statistics. J Stat Softw. 2009;31:1–21.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

