In vitro and in vivo study of additive manufactured porous Ti6Al4V scaffolds for repairing bone defects
- PMID: 27667204
- PMCID: PMC5036184
- DOI: 10.1038/srep34072
In vitro and in vivo study of additive manufactured porous Ti6Al4V scaffolds for repairing bone defects
Abstract
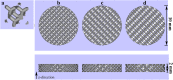
Metallic implants with a low effective modulus can provide early load-bearing and reduce stress shielding, which is favorable for increasing in vivo life-span. In this research, porous Ti6Al4V scaffolds with three pore sizes (300~400, 400~500, and 500~700 μm) were manufactured by Electron Beam Melting, with an elastic modulus range of 3.7 to 1.7 GPa. Cytocompatibility in vitro and osseointegration ability in vivo of scaffolds were assessed. hBMSCs numbers increased on all porous scaffolds over time. The group with intended pore sizes of 300 to 400 μm was significantly higher than that of the other two porous scaffolds at days 5 and 7. This group also had higher ALP activity at day 7 in osteogenic differentiation experiment. The scaffold with pore size of 300 to 400 μm was implanted into a 30-mm segmental defect of goat metatarsus. In vivo evaluations indicated that the depth of bone ingrowth increased over time and no implant dislocation occurred during the experiment. Based on its better cytocompatibility and favorable bone ingrowth, the present data showed the capability of the additive manufactured porous Ti6Al4V scaffold with an intended pore size of 300 to 400 μm for large segmental bone defects.
Figures






References
-
- De Long W. G. Jr. et al. Bone grafts and bone graft substitutes in orthopaedic trauma surgery. A critical analysis. J. Bone Joint Surg. Am. 89, 649–658 (2007). - PubMed
-
- Kurien T., Pearson R. G. & Scammell B. E. Bone graft substitutes currently available in orthopaedic practice: the evidence for their use. Bone Joint J. 95B, 583–597 (2013). - PubMed
-
- LeGeros R. Z. Properties of osteoconductive biomaterials: calcium phosphates. Clin. Orthop. Relat. Res. 395, 81–98 (2002). - PubMed
-
- Brånemark P. I. et al. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand. J. Plast Reconstr. Surg. Suppl. 16, 132 (1977). - PubMed
-
- Khan S. N., Tomin E. & Lane J. M. Clinical applications of bone graft substitutes. Orthop. Clin. North Am. 31, 389–398 (2000). - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

