Role of End Binding Protein-1 in endothelial permeability response to barrier-disruptive and barrier-enhancing agonists
- PMID: 27667566
- PMCID: PMC5138117
- DOI: 10.1016/j.cellsig.2016.09.005
Role of End Binding Protein-1 in endothelial permeability response to barrier-disruptive and barrier-enhancing agonists
Abstract
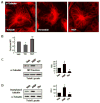
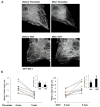
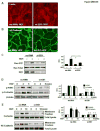
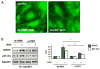
Rapid changes in microtubule (MT) polymerization dynamics affect regional activity of small GTPases RhoA and Rac1, which play a key role in the regulation of actin cytoskeleton and endothelial cell (EC) permeability. This study tested the role of End Binding Protein-1 (EB1) in the mechanisms of increased and decreased EC permeability caused by thrombin and hepatocyte growth factor (HGF) and mediated by RhoA and Rac1 GTPases, respectively. Stimulation of human lung EC with thrombin inhibited peripheral MT growth, which was monitored by morphological and biochemical evaluation of peripheral MT and the levels of stabilized MT. In contrast, stimulation of EC with HGF promoted peripheral MT growth and protrusion of EB1-positive MT plus ends to the EC peripheral submembrane area. EB1 knockdown by small interfering RNA did not affect partial MT depolymerization, activation of Rho signaling, and permeability response to thrombin, but suppressed the HGF-induced endothelial barrier enhancement. EB1 knockdown suppressed HGF-induced activation of Rac1 and Rac1 cytoskeletal effectors cortactin and PAK1, impaired HGF-induced assembly of cortical cytoskeleton regulatory complex (WAVE-p21Arc-IQGAP1), and blocked HGF-induced enhancement of peripheral actin cytoskeleton and VE-cadherin-positive adherens junctions. Altogether, these data demonstrate a role for EB1 in coordination of MT-dependent barrier enhancement response to HGF, but show no involvement of EB1 in acute increase of EC permeability caused by the barrier disruptive agonist. The results suggest that increased peripheral EB1 distribution is a critical component of the Rac1-mediated pathway and peripheral cytoskeletal remodeling essential for agonist-induced EC barrier enhancement.
Keywords: Cytoskeleton; GTPase; Microtubule dynamics; Permeability; Pulmonary endothelium.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
IQGAP1 regulates endothelial barrier function via EB1-cortactin cross talk.Mol Cell Biol. 2014 Sep 15;34(18):3546-58. doi: 10.1128/MCB.00248-14. Epub 2014 Jul 14. Mol Cell Biol. 2014. PMID: 25022754 Free PMC article.
-
HGF attenuates thrombin-induced endothelial permeability by Tiam1-mediated activation of the Rac pathway and by Tiam1/Rac-dependent inhibition of the Rho pathway.FASEB J. 2007 Sep;21(11):2776-86. doi: 10.1096/fj.06-7660com. Epub 2007 Apr 11. FASEB J. 2007. PMID: 17428964
-
Hepatocyte growth factor-induced Asef-IQGAP1 complex controls cytoskeletal remodeling and endothelial barrier.J Biol Chem. 2015 Feb 13;290(7):4097-109. doi: 10.1074/jbc.M114.620377. Epub 2014 Dec 9. J Biol Chem. 2015. PMID: 25492863 Free PMC article.
-
Driving Rho GTPase activity in endothelial cells regulates barrier integrity.Thromb Haemost. 2010 Jan;103(1):40-55. doi: 10.1160/TH09-06-0403. Epub 2009 Sep 30. Thromb Haemost. 2010. PMID: 20062930 Review.
-
Cytoskeletal dynamics and lung fluid balance.Compr Physiol. 2012 Jan;2(1):449-78. doi: 10.1002/cphy.c100006. Compr Physiol. 2012. PMID: 23728978 Review.
Cited by
-
HGF/Met Signaling in Cancer Invasion: The Impact on Cytoskeleton Remodeling.Cancers (Basel). 2017 May 5;9(5):44. doi: 10.3390/cancers9050044. Cancers (Basel). 2017. PMID: 28475121 Free PMC article. Review.
-
Septin and actin contributions to endothelial cell-cell junctions and monolayer integrity.Cytoskeleton (Hoboken). 2023 Jul-Aug;80(7-8):228-241. doi: 10.1002/cm.21732. Epub 2022 Oct 17. Cytoskeleton (Hoboken). 2023. PMID: 36205643 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

