Osteoblast Differentiation at a Glance
- PMID: 27667570
- PMCID: PMC5040224
- DOI: 10.12659/msmbr.901142
Osteoblast Differentiation at a Glance
Abstract
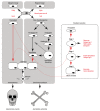
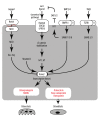
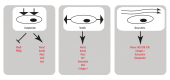
Ossification is a tightly regulated process, performed by specialized cells called osteoblasts. Dysregulation of this process may cause inadequate or excessive mineralization of bones or ectopic calcification, all of which have grave consequences for human health. Understanding osteoblast biology may help to treat diseases such as osteogenesis imperfecta, calcific heart valve disease, osteoporosis, and many others. Osteoblasts are bone-building cells of mesenchymal origin; they differentiate from mesenchymal progenitors, either directly or via an osteochondroprogenitor. The direct pathway is typical for intramembranous ossification of the skull and clavicles, while the latter is a hallmark of endochondral ossification of the axial skeleton and limbs. The pathways merge at the level of preosteoblasts, which progress through 3 stages: proliferation, matrix maturation, and mineralization. Osteoblasts can also differentiate into osteocytes, which are stellate cells populating narrow interconnecting passages within the bone matrix. The key molecular switch in the commitment of mesenchymal progenitors to osteoblast lineage is the transcription factor cbfa/runx2, which has multiple upstream regulators and a wide variety of targets. Upstream is the Wnt/Notch system, Sox9, Msx2, and hedgehog signaling. Cofactors of Runx2 include Osx, Atf4, and others. A few paracrine and endocrine factors serve as coactivators, in particular, bone morphogenetic proteins and parathyroid hormone. The process is further fine-tuned by vitamin D and histone deacetylases. Osteoblast differentiation is subject to regulation by physical stimuli to ensure the formation of bone adequate for structural and dynamic support of the body. Here, we provide a brief description of the various stimuli that influence osteogenesis: shear stress, compression, stretch, micro- and macrogravity, and ultrasound. A complex understanding of factors necessary for osteoblast differentiation paves a way to introduction of artificial bone matrices.
Figures
References
-
- Papachroni KK, Karatzas DN, Papavassiliou KA, et al. Mechanotransduction in osteoblast regulation and bone disease. Trends Mol Med. 2009;15(5):208–16. - PubMed
-
- Hernlund E, Svedbom A, Ivergard M, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA) Arch Osteoporos. 2013;8:136. - PMC - PubMed
-
- Quarto R, Mastrogiacomo M, Cancedda R, et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344(5):385–86. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

