Tolerogenic dendritic cells generated with dexamethasone and vitamin D3 regulate rheumatoid arthritis CD4+ T cells partly via transforming growth factor-β1
- PMID: 27667787
- PMCID: PMC5167049
- DOI: 10.1111/cei.12870
Tolerogenic dendritic cells generated with dexamethasone and vitamin D3 regulate rheumatoid arthritis CD4+ T cells partly via transforming growth factor-β1
Abstract
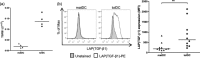
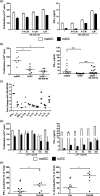
Tolerogenic dendritic cells (tolDC) are a new immunotherapeutic tool for the treatment of rheumatoid arthritis (RA) and other autoimmune disorders. We have established a method to generate stable tolDC by pharmacological modulation of human monocyte-derived DC. These tolDC exert potent pro-tolerogenic actions on CD4+ T cells. Lack of interleukin (IL)-12p70 production is a key immunoregulatory attribute of tolDC but does not explain their action fully. Here we show that tolDC express transforming growth factor (TGF)-β1 at both mRNA and protein levels, and that expression of this immunoregulatory cytokine is significantly higher in tolDC than in mature monocyte-derived DC. By inhibiting TGF-β1 signalling we demonstrate that tolDC regulate CD4+ T cell responses in a manner that is at least partly dependent upon this cytokine. Crucially, we also show that while there is no significant difference in expression of TGF-βRII on CD4+ T cells from RA patients and healthy controls, RA patient CD4+ T cells are measurably less responsive to TGF-β1 than healthy control CD4+ T cells [reduced TGF-β-induced mothers against decapentaplegic homologue (Smad)2/3 phosphorylation, forkhead box protein 3 (FoxP3) expression and suppression of (IFN)-γ secretion]. However, CD4+ T cells from RA patients can, nonetheless, be regulated efficiently by tolDC in a TGF-β1-dependent manner. This work is important for the design and development of future studies investigating the potential use of tolDC as a novel immunotherapy for the treatment of RA.
Keywords: TGF-β1; regulation; rheumatoid arthritis; tolerogenic dendritic cells.
© 2016 British Society for Immunology.
Figures


Similar articles
-
Induction of porcine-specific regulatory T cells with high specificity and expression of IL-10 and TGF-β1 using baboon-derived tolerogenic dendritic cells.Xenotransplantation. 2018 Jan;25(1). doi: 10.1111/xen.12355. Epub 2017 Oct 22. Xenotransplantation. 2018. PMID: 29057512
-
Targeting of tolerogenic dendritic cells to heat-shock proteins in inflammatory arthritis.J Transl Med. 2019 Nov 14;17(1):375. doi: 10.1186/s12967-019-2128-4. J Transl Med. 2019. PMID: 31727095 Free PMC article.
-
Generation and characterisation of therapeutic tolerogenic dendritic cells for rheumatoid arthritis.Ann Rheum Dis. 2010 Nov;69(11):2042-50. doi: 10.1136/ard.2009.126383. Epub 2010 Jun 15. Ann Rheum Dis. 2010. PMID: 20551157 Free PMC article.
-
Tolerogenic dendritic cell therapy for rheumatoid arthritis: where are we now?Clin Exp Immunol. 2013 May;172(2):148-57. doi: 10.1111/cei.12038. Clin Exp Immunol. 2013. PMID: 23574312 Free PMC article. Review.
-
Developing tolerogenic dendritic cell therapy for rheumatoid arthritis: what can we learn from mouse models?Ann Rheum Dis. 2011 Sep;70(9):1526-33. doi: 10.1136/ard.2011.151654. Ann Rheum Dis. 2011. PMID: 21804099 Review.
Cited by
-
Induction of Tolerogenic Dendritic Cells by Endogenous Biomolecules: An Update.Front Immunol. 2018 Oct 26;9:2482. doi: 10.3389/fimmu.2018.02482. eCollection 2018. Front Immunol. 2018. PMID: 30416505 Free PMC article. Review.
-
Tolerogenic dendritic cells generated in vitro using a novel protocol mimicking mucosal tolerance mechanisms represent a potential therapeutic cell platform for induction of immune tolerance.Front Immunol. 2023 Oct 13;14:1045183. doi: 10.3389/fimmu.2023.1045183. eCollection 2023. Front Immunol. 2023. PMID: 37901231 Free PMC article.
-
Vitamin D, Autoimmune Disease and Rheumatoid Arthritis.Calcif Tissue Int. 2020 Jan;106(1):58-75. doi: 10.1007/s00223-019-00577-2. Epub 2019 Jul 8. Calcif Tissue Int. 2020. PMID: 31286174 Free PMC article. Review.
-
Development and Functional Characterization of Murine Tolerogenic Dendritic Cells.J Vis Exp. 2018 May 18;(135):57637. doi: 10.3791/57637. J Vis Exp. 2018. PMID: 29863666 Free PMC article.
-
Tolerogenic dendritic cell reporting: Has a minimum information model made a difference?PeerJ. 2023 May 31;11:e15352. doi: 10.7717/peerj.15352. eCollection 2023. PeerJ. 2023. PMID: 37273539 Free PMC article.
References
-
- Boissier MC, Semerano L, Challal S, Saidenberg‐Kermanac'h N, Falgarone G. Rheumatoid arthritis: from autoimmunity to synovitis and joint destruction. J Autoimmun 2012; 39:222–8. - PubMed
-
- Stoop JN, Harry RA, von Delwig A, Isaacs JD, Robinson JH, Hilkens CM. Therapeutic effect of tolerogenic dendritic cells in established collagen‐induced arthritis is associated with a reduction in Th17 responses. Arthritis Rheum 2010; 62:3656–65. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

