Glycosylation and stem cells: Regulatory roles and application of iPSCs in the study of glycosylation-related disorders
- PMID: 27667795
- PMCID: PMC5214967
- DOI: 10.1002/bies.201600138
Glycosylation and stem cells: Regulatory roles and application of iPSCs in the study of glycosylation-related disorders
Abstract
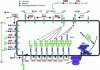
Glycosylation refers to the co- and post-translational modification of protein and lipids by monosaccharides or oligosaccharide chains. The surface of mammalian cells is decorated by a heterogeneous and highly complex array of protein and lipid linked glycan structures that vary significantly between different cell types, raising questions about their roles in development and disease pathogenesis. This review will begin by focusing on recent findings that define roles for cell surface protein and lipid glycosylation in pluripotent stem cells and their functional impact during normal development. Then, we will describe how patient derived induced pluripotent stem cells are being used to model human diseases such as congenital disorders of glycosylation. Collectively, these studies indicate that cell surface glycans perform critical roles in human development and disease.
Keywords: congenital disorders of glycosylation; glycosylation; pluripotent stem cells.
© 2016 WILEY Periodicals, Inc.
Figures


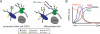

Similar articles
-
Glycomic Characterization of Induced Pluripotent Stem Cells Derived from a Patient Suffering from Phosphomannomutase 2 Congenital Disorder of Glycosylation (PMM2-CDG).Mol Cell Proteomics. 2016 Apr;15(4):1435-52. doi: 10.1074/mcp.M115.054122. Epub 2016 Jan 19. Mol Cell Proteomics. 2016. PMID: 26785728 Free PMC article.
-
Structural Changes in N-Glycans on Induced Pluripotent Stem Cells Differentiating Toward Cardiomyocytes.Stem Cells Transl Med. 2015 Nov;4(11):1258-64. doi: 10.5966/sctm.2015-0029. Epub 2015 Sep 16. Stem Cells Transl Med. 2015. PMID: 26378261 Free PMC article.
-
Congenital disorders of glycosylation: genetic model systems lead the way.Trends Cell Biol. 2001 Mar;11(3):136-41. doi: 10.1016/s0962-8924(01)01925-0. Trends Cell Biol. 2001. PMID: 11306275 Review.
-
Modeling Inborn Errors of Hepatic Metabolism Using Induced Pluripotent Stem Cells.Arterioscler Thromb Vasc Biol. 2017 Nov;37(11):1994-1999. doi: 10.1161/ATVBAHA.117.309199. Epub 2017 Aug 17. Arterioscler Thromb Vasc Biol. 2017. PMID: 28818857 Free PMC article. Review.
-
Reference glycan structure libraries of primary human cardiomyocytes and pluripotent stem cell-derived cardiomyocytes reveal cell-type and culture stage-specific glycan phenotypes.J Mol Cell Cardiol. 2020 Feb;139:33-46. doi: 10.1016/j.yjmcc.2019.12.012. Epub 2020 Jan 21. J Mol Cell Cardiol. 2020. PMID: 31972267 Free PMC article.
Cited by
-
The Warburg effect is necessary to promote glycosylation in the blastema during zebrafish tail regeneration.NPJ Regen Med. 2021 Sep 13;6(1):55. doi: 10.1038/s41536-021-00163-x. NPJ Regen Med. 2021. PMID: 34518542 Free PMC article.
-
A Rapid Array-Based Approach to N-Glycan Profiling of Cultured Cells.J Proteome Res. 2019 Oct 4;18(10):3630-3639. doi: 10.1021/acs.jproteome.9b00303. Epub 2019 Sep 19. J Proteome Res. 2019. PMID: 31535553 Free PMC article.
-
Importance of evaluating protein glycosylation in pluripotent stem cell-derived cardiomyocytes for research and clinical applications.Pflugers Arch. 2021 Jul;473(7):1041-1059. doi: 10.1007/s00424-021-02554-x. Epub 2021 Apr 8. Pflugers Arch. 2021. PMID: 33830329 Free PMC article. Review.
-
Representing glycophenotypes: semantic unification of glycobiology resources for disease discovery.Database (Oxford). 2019 Jan 1;2019:baz114. doi: 10.1093/database/baz114. Database (Oxford). 2019. PMID: 31735951 Free PMC article. Review.
-
Emerging roles of N-linked glycosylation in brain physiology and disorders.Trends Endocrinol Metab. 2021 Dec;32(12):980-993. doi: 10.1016/j.tem.2021.09.006. Epub 2021 Oct 29. Trends Endocrinol Metab. 2021. PMID: 34756776 Free PMC article. Review.
References
-
- Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

