Screening for chromosomal abnormalities using combined test in the first trimester of pregnancy
- PMID: 27668198
- PMCID: PMC5028642
- DOI: 10.5468/ogs.2016.59.5.357
Screening for chromosomal abnormalities using combined test in the first trimester of pregnancy
Abstract
Objective: This study was designed to review the screening performance of combined test at the Ewha Womans University Mokdong hospital.
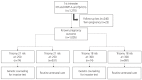
Methods: All women admitted for routine antenatal care between January 1st 2008 and December 31st 2012 with a known pregnancy outcome were included in this study, totaling 1,156 women with singleton pregnancies presenting at 10 to 13 weeks of gestation. Women were offered screening using a combination of maternal serum pregnancy-associated plasma protein-A, free β-human chorionic gonadotropin and fetal nuchal translucency thickness. Those with an estimated risk of ≥1 in 250 of carrying a fetus with trisomy 21 or ≥1 in 300 risk of trisomy 18 were offered genetic counseling with the option of an invasive diagnostic test.
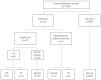
Results: The median of gestational age was 11+3 weeks, the median of crown-rump length was 47.1 mm, and the median age of the women was 31 years. The detection rate was 80% for trisomy 21 (4 of 5) and 100% for trisomy 13 and 18 (all 2). The false-positive rate was 7.73% for trisomy 21 and 1.21% for trisomy 18.
Conclusion: This study was the first large population study performed with the aim of analyzing the performance of the combined test in Korea. This study demonstrated that the detection rates and other figures of the first trimester combined test are comparable to the results reported in other papers worldwide. Consequently, if strict conditions for good screening outcomes are achieved, the first trimester combined test might well be the earliest detectable screening, improving detection rates without increasing karyotyping or economic and other implications that inevitably ensue.
Keywords: Combined test; Down screening; Fetal aneuploidy; First trimester screening; Prenatal diagnosis.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures


References
-
- ACOG Committee on Practice Bulletins. ACOG Practice Bulletin No. 77: screening for fetal chromosomal abnormalities. Obstet Gynecol. 2007;109:217–227. - PubMed
-
- Schuchter K, Hafner E, Stangl G, Metzenbauer M, Hofinger D, Philipp K. The first trimester 'combined test' for the detection of Down syndrome pregnancies in 4939 unselected pregnancies. Prenat Diagn. 2002;22:211–215. - PubMed
-
- Wald NJ, Hackshaw AK. Combining ultrasound and biochemistry in first-trimester screening for Down's syndrome. Prenat Diagn. 1997;17:821–829. - PubMed
-
- Spencer K, Spencer CE, Power M, Moakes A, Nicolaides KH. One stop clinic for assessment of risk for fetal anomalies: a report of the first year of prospective screening for chromosomal anomalies in the first trimester. BJOG. 2000;107:1271–1275. - PubMed
-
- Spencer K, Spencer CE, Power M, Dawson C, Nicolaides KH. Screening for chromosomal abnormalities in the first trimester using ultrasound and maternal serum biochemistry in a one-stop clinic: a review of three years prospective experience. BJOG. 2003;110:281–286. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

