Protein sequences bound to mineral surfaces persist into deep time
- PMID: 27668515
- PMCID: PMC5039028
- DOI: 10.7554/eLife.17092
Protein sequences bound to mineral surfaces persist into deep time
Abstract
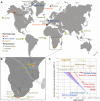
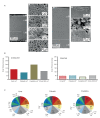
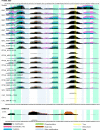
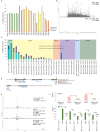
Proteins persist longer in the fossil record than DNA, but the longevity, survival mechanisms and substrates remain contested. Here, we demonstrate the role of mineral binding in preserving the protein sequence in ostrich (Struthionidae) eggshell, including from the palaeontological sites of Laetoli (3.8 Ma) and Olduvai Gorge (1.3 Ma) in Tanzania. By tracking protein diagenesis back in time we find consistent patterns of preservation, demonstrating authenticity of the surviving sequences. Molecular dynamics simulations of struthiocalcin-1 and -2, the dominant proteins within the eggshell, reveal that distinct domains bind to the mineral surface. It is the domain with the strongest calculated binding energy to the calcite surface that is selectively preserved. Thermal age calculations demonstrate that the Laetoli and Olduvai peptides are 50 times older than any previously authenticated sequence (equivalent to ~16 Ma at a constant 10°C).
Keywords: Struthio camelus; biochemistry; biomineralization; eggshell; evolutionary biology; genomics; molecular dynamics; paleontology; paleoproteomics.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures




Comment in
- doi: 10.7554/eLife.20877
Similar articles
-
Survival of mineral-bound peptides into the Miocene.Elife. 2022 Dec 19;11:e82849. doi: 10.7554/eLife.82849. Elife. 2022. PMID: 36533893 Free PMC article.
-
Proteins from the past.Elife. 2016 Sep 27;5:e20877. doi: 10.7554/eLife.20877. Elife. 2016. PMID: 27668514 Free PMC article.
-
Structure of struthiocalcin-1, an intramineral protein from Struthio camelus eggshell, in two crystal forms.Acta Crystallogr D Biol Crystallogr. 2015 Apr;71(Pt 4):809-18. doi: 10.1107/S139900471500125X. Epub 2015 Mar 26. Acta Crystallogr D Biol Crystallogr. 2015. PMID: 25849392
-
In situ ∼2.0 Ma trees discovered as fossil rooted stumps, lowermost Bed I, Olduvai Gorge, Tanzania.J Hum Evol. 2016 Jan;90:74-87. doi: 10.1016/j.jhevol.2015.09.011. Epub 2015 Nov 23. J Hum Evol. 2016. PMID: 26767961
-
Comparing apples and oranges in the Plio-Pleistocene: methodological comments on "meat-eating by early hominids at the FLK 22 Zinjanthropus site, Olduvai Gorge (Tanzania): an experimental approach using cut-mark data".J Hum Evol. 1999 Nov;37(5):789-92; discussion 793-800. doi: 10.1006/jhev.1999.0320. J Hum Evol. 1999. PMID: 10536091 Review. No abstract available.
Cited by
-
Faecal proteomics as a novel method to study mammalian behaviour and physiology.Mol Ecol Resour. 2021 Aug;21(6):1808-1819. doi: 10.1111/1755-0998.13380. Epub 2021 Apr 8. Mol Ecol Resour. 2021. PMID: 33720532 Free PMC article.
-
Tooth Enamel and its Dynamic Protein Matrix.Int J Mol Sci. 2020 Jun 23;21(12):4458. doi: 10.3390/ijms21124458. Int J Mol Sci. 2020. PMID: 32585904 Free PMC article. Review.
-
Bayesian Total-Evidence Dating Revisits Sloth Phylogeny and Biogeography: A Cautionary Tale on Morphological Clock Analyses.Syst Biol. 2024 May 27;73(1):125-139. doi: 10.1093/sysbio/syad069. Syst Biol. 2024. PMID: 38041854 Free PMC article.
-
Paleoproteomic profiling of organic residues on prehistoric pottery from Malta.Amino Acids. 2021 Feb;53(2):295-312. doi: 10.1007/s00726-021-02946-4. Epub 2021 Feb 13. Amino Acids. 2021. PMID: 33582869 Free PMC article.
-
Reconstructing medieval diets through the integration of stable isotope and proteomic analyses from two European burial sites.Sci Rep. 2025 Jul 21;15(1):26442. doi: 10.1038/s41598-025-10103-0. Sci Rep. 2025. PMID: 40691239 Free PMC article.
References
-
- Abdou AM, Sato K, Kim M. Bioactive Food Peptides in Health and Disease. INTECH Open Access Publisher; 2013. Functional proteins and peptides of hen’s egg origin. - DOI
-
- Allentoft ME, Collins M, Harker D, Haile J, Oskam CL, Hale ML, Campos PF, Samaniego JA, Gilbert MTP, Willerslev E, Zhang G, Scofield RP, Holdaway RN, Bunce M. The half-life of DNA in bone: measuring decay kinetics in 158 dated fossils. Proceedings of the Royal Society B. 2012;279:4724–4733. doi: 10.1098/rspb.2012.1745. - DOI - PMC - PubMed
-
- Aoki N, Sakiyama A, Kuroki K, Maenaka K, Kohda D, Deshimaru M, Terada S. Serotriflin, a CRISP family protein with binding affinity for small serum protein-2 in snake serum. Biochimica Et Biophysica Acta (BBA) - Proteins and Proteomics. 2008;1784:621–628. doi: 10.1016/j.bbapap.2007.12.010. - DOI - PubMed
-
- Ballantyne AP, Greenwood DR, Sinninghe Damste JS, Csank AZ, Eberle JJ, Rybczynski N. Significantly warmer Arctic surface temperatures during the Pliocene indicated by multiple independent proxies. Geology. 2010;38:603–606. doi: 10.1130/G30815.1. - DOI

