Protein sequences bound to mineral surfaces persist into deep time
- PMID: 27668515
- PMCID: PMC5039028
- DOI: 10.7554/eLife.17092
Protein sequences bound to mineral surfaces persist into deep time
Abstract
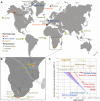
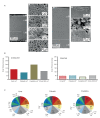
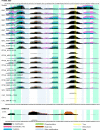
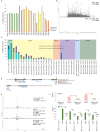
Proteins persist longer in the fossil record than DNA, but the longevity, survival mechanisms and substrates remain contested. Here, we demonstrate the role of mineral binding in preserving the protein sequence in ostrich (Struthionidae) eggshell, including from the palaeontological sites of Laetoli (3.8 Ma) and Olduvai Gorge (1.3 Ma) in Tanzania. By tracking protein diagenesis back in time we find consistent patterns of preservation, demonstrating authenticity of the surviving sequences. Molecular dynamics simulations of struthiocalcin-1 and -2, the dominant proteins within the eggshell, reveal that distinct domains bind to the mineral surface. It is the domain with the strongest calculated binding energy to the calcite surface that is selectively preserved. Thermal age calculations demonstrate that the Laetoli and Olduvai peptides are 50 times older than any previously authenticated sequence (equivalent to ~16 Ma at a constant 10°C).
Keywords: Struthio camelus; biochemistry; biomineralization; eggshell; evolutionary biology; genomics; molecular dynamics; paleontology; paleoproteomics.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures




Comment in
- doi: 10.7554/eLife.20877
References
-
- Abdou AM, Sato K, Kim M. Bioactive Food Peptides in Health and Disease. INTECH Open Access Publisher; 2013. Functional proteins and peptides of hen’s egg origin. - DOI
-
- Allentoft ME, Collins M, Harker D, Haile J, Oskam CL, Hale ML, Campos PF, Samaniego JA, Gilbert MTP, Willerslev E, Zhang G, Scofield RP, Holdaway RN, Bunce M. The half-life of DNA in bone: measuring decay kinetics in 158 dated fossils. Proceedings of the Royal Society B. 2012;279:4724–4733. doi: 10.1098/rspb.2012.1745. - DOI - PMC - PubMed
-
- Aoki N, Sakiyama A, Kuroki K, Maenaka K, Kohda D, Deshimaru M, Terada S. Serotriflin, a CRISP family protein with binding affinity for small serum protein-2 in snake serum. Biochimica Et Biophysica Acta (BBA) - Proteins and Proteomics. 2008;1784:621–628. doi: 10.1016/j.bbapap.2007.12.010. - DOI - PubMed
-
- Ballantyne AP, Greenwood DR, Sinninghe Damste JS, Csank AZ, Eberle JJ, Rybczynski N. Significantly warmer Arctic surface temperatures during the Pliocene indicated by multiple independent proxies. Geology. 2010;38:603–606. doi: 10.1130/G30815.1. - DOI

