Variation in Private Payer Coverage of Rheumatoid Arthritis Drugs
- PMID: 27668566
- PMCID: PMC10397684
- DOI: 10.18553/jmcp.2016.22.10.1176
Variation in Private Payer Coverage of Rheumatoid Arthritis Drugs
Abstract
Background: Payers in the United States issue coverage determinations to guide how their enrolled beneficiaries use prescription drugs. Because payers create their own coverage policies, how they cover drugs can vary, which in turn can affect access to care by beneficiaries.
Objective: To examine how the largest private payers based on membership cover drugs indicated for rheumatoid arthritis and to determine what evidence the payers reported reviewing when formulating their coverage policies.
Methods: Coverage policies issued by the 10 largest private payers that make their policies publicly available were identified for rheumatoid arthritis drugs. Each coverage determination was compared with the drug's corresponding FDA label and categorized according to the following: (a) consistent with the label, (b) more restrictive than the label, (c) less restrictive than the label, or (d) mixed (i.e., more restrictive than the label in one way but less restrictive in another). Each coverage determination was also compared with the American College of Rheumatology (ACR) 2012 treatment recommendations and categorized using the same relative restrictiveness criteria. The policies were then reviewed to identify the evidence that the payers reported reviewing. The identified evidence was divided into the following 6 categories: randomized controlled trials; other clinical studies (e.g., observational studies); health technology assessments; clinical reviews; cost-effectiveness analyses; and clinical guidelines.
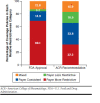
Results: Sixty-nine percent of coverage determinations were more restrictive than the corresponding FDA label; 15% were consistent; 3% were less restrictive; and 13% were mixed. Thirty-four percent of coverage determinations were consistent with the ACR recommendations, 33% were more restrictive; 17% were less restrictive; and 17% were mixed. Payers most often reported reviewing randomized controlled trials for their coverage policies (an average of 2.3 per policy). The payers reported reviewing an average of 1.4 clinical guidelines, 1.1 clinical reviews, 0.8 other clinical studies, and 0.5 technology assessments per policy. Only 1 payer reported reviewing cost-effectiveness analyses. The evidence base that the payers reported reviewing varied in terms of volume and composition.
Conclusions: Payers most often covered rheumatoid arthritis drugs more restrictively than the corresponding FDA label indication and the ACR treatment recommendations. Payers reported reviewing a varied evidence base in their coverage policies.
Disclosures: Funding for this study was provided by Genentech. Chambers has participated in a Sanofi advisory board, unrelated to this study. The authors report no other potential conflicts of interest. Study concept and design were contributed by Chambers. Anderson, Wilkinson, and Chenoweth collected the data, assisted by Chambers, and data interpretation was primarily performed by Chambers, along with Anderson and with assistance from Wilkinson and Chenoweth. The manuscript was written primarily by Chambers, along with Wilkinson and with assistance from Anderson and Chenoweth. Chambers, Chenoweth, Wilkinson, and Anderson revised the manuscript.
Conflict of interest statement
Funding for this study was provided by Genentech. Chambers has participated in a Sanofi advisory board, unrelated to this study. The authors report no other potential conflicts of interest.
Study concept and design were contributed by Chambers. Anderson, Wilkinson, and Chenoweth collected the data, assisted by Chambers, and data interpretation was primarily performed by Chambers, along with Anderson and with assistance from Wilkinson and Chenoweth. The manuscript was written primarily by Chambers, along with Wilkinson and with assistance from Anderson and Chenoweth. Chambers, Chenoweth, Wilkinson, and Anderson revised the manuscript.
Figures
References
-
- Chambers JD, Chenoweth MD, Pyo J, Cangelosi MJ, Neumann PJ.. Changing face of Medicare's National Coverage Determinations for technology. Int J Technol Assess Health Care. 2015;31(5):347-54. - PubMed
-
- Chambers JD, Chenoweth M, Cangelosi MJ, Pyo J, Cohen JT, Neumann PJ.. Medicare is scrutinizing evidence more tightly for national coverage determinations. Health Aff (Millwood). 2015;34(2):253-60. - PubMed
-
- Meckley LM, Neumann PJ.. Personalized medicine: factors influencing reimbursement. Health Policy. 2010;94(2):91-100. - PubMed
-
- Chambers JD, Morris S, Neumann PJ, Buxton MJ.. Factors predicting Medicare national coverage: an empirical analysis. Med Care. 2012;50(3):249-56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical