Fundus Autofluorescence in Age-related Macular Degeneration
- PMID: 27668639
- PMCID: PMC5287441
- DOI: 10.1097/OPX.0000000000000997
Fundus Autofluorescence in Age-related Macular Degeneration
Abstract
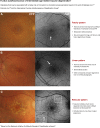
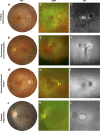
: Fundus autofluorescence (FAF) provides detailed insight into the health of the retinal pigment epithelium (RPE). This is highly valuable in age-related macular degeneration (AMD) as RPE damage is a hallmark of the disease. The purpose of this paper is to critically appraise current clinical descriptions regarding the appearance of AMD using FAF and to integrate these findings into a chair-side reference. A wide variety of FAF patterns have been described in AMD, which is consistent with the clinical heterogeneity of the disease. In particular, FAF imaging in early to intermediate AMD has the capacity to reveal RPE alterations in areas that appear normal on funduscopy, which aids in the stratification of cases and may have visually significant prognostic implications. It can assist in differential diagnoses and also represents a reliable, sensitive method for distinguishing reticular pseudodrusen. FAF is especially valuable in the detection, evaluation, and monitoring of geographic atrophy and has been used as an endpoint in clinical trials. In neovascular AMD, FAF reveals distinct patterns of classic choroidal neovascularization noninvasively and may be especially useful for determining which eyes are likely to benefit from therapeutic intervention. FAF represents a rapid, effective, noninvasive imaging method that has been underutilized, and incorporation into the routine assessment of AMD cases should be considered. However, the practicing clinician should also be aware of the limitations of the modality, such as in the detection of foveal involvement and in the distinction of phenotypes (hypo-autofluorescent drusen from small areas of geographic atrophy).
Figures




References
-
- Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014;2:e106–16. - PubMed
-
- Nivison-Smith L, Milston R, Madigan M, et al. Age-related macular degeneration: linking clinical presentation to pathology. Optom Vis Sci 2014;91:832–48. - PubMed
-
- American Optometric Association Consensus Panel on Care of the Patient with Age-Related Macular Degeneration. Optometric Clinical Practice Guideline. Care of the Patient with Age-Related Macular Degeneration; 2004. Available at: http://www.aoa.org/documents/optometrists/CPG-6.pdf. Accessed: January 4, 2016.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

