A Carboxylate Shift Regulates Dioxygen Activation by the Diiron Nonheme β-Hydroxylase CmlA upon Binding of a Substrate-Loaded Nonribosomal Peptide Synthetase
- PMID: 27668828
- PMCID: PMC5258830
- DOI: 10.1021/acs.biochem.6b00834
A Carboxylate Shift Regulates Dioxygen Activation by the Diiron Nonheme β-Hydroxylase CmlA upon Binding of a Substrate-Loaded Nonribosomal Peptide Synthetase
Abstract
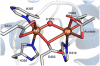
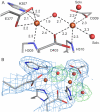
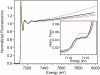
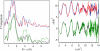
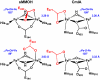
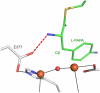
The first step in the nonribosomal peptide synthetase (NRPS)-based biosynthesis of chloramphenicol is the β-hydroxylation of the precursor l-p-aminophenylalanine (l-PAPA) catalyzed by the monooxygenase CmlA. The active site of CmlA contains a dinuclear iron cluster that is reduced to the diferrous state (WTR) to initiate O2 activation. However, rapid O2 activation occurs only when WTR is bound to CmlP, the NRPS to which l-PAPA is covalently attached. Here the X-ray crystal structure of WTR is reported, which is very similar to that of the as-isolated diferric enzyme in which the irons are coordinately saturated. X-ray absorption spectroscopy is used to investigate the WTR cluster ligand structure as well as the structures of WTR in complex with a functional CmlP variant (CmlPAT) with and without l-PAPA attached. It is found that formation of the active WTR:CmlPAT-l-PAPA complex converts at least one iron of the cluster from six- to five-coordinate by changing a bidentately bound amino acid carboxylate to monodentate on Fe1. The only bidentate carboxylate in the structure of WTR is E377. The crystal structure of the CmlA variant E377D shows only monodentate carboxylate coordination. Reduced E377D reacts rapidly with O2 in the presence or absence of CmlPAT-l-PAPA, showing loss of regulation. However, this variant fails to catalyze hydroxylation, suggesting that E377 has the dual role of coupling regulation of O2 reactivity with juxtaposition of the substrate and the reactive oxygen species. The carboxylate shift in response to substrate binding represents a novel regulatory strategy for oxygen activation in diiron oxygenases.
Figures



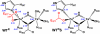
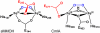
Similar articles
-
A family of diiron monooxygenases catalyzing amino acid beta-hydroxylation in antibiotic biosynthesis.Proc Natl Acad Sci U S A. 2010 Aug 31;107(35):15391-6. doi: 10.1073/pnas.1007953107. Epub 2010 Aug 16. Proc Natl Acad Sci U S A. 2010. PMID: 20713732 Free PMC article.
-
Diiron monooxygenases in natural product biosynthesis.Nat Prod Rep. 2018 Jul 18;35(7):646-659. doi: 10.1039/C7NP00061H. Nat Prod Rep. 2018. PMID: 29552683 Free PMC article. Review.
-
Structure of a dinuclear iron cluster-containing β-hydroxylase active in antibiotic biosynthesis.Biochemistry. 2013 Sep 24;52(38):6662-71. doi: 10.1021/bi400845b. Epub 2013 Sep 11. Biochemistry. 2013. PMID: 23980641 Free PMC article.
-
Oxygen activation by nonheme iron(II) complexes: alpha-keto carboxylate versus carboxylate.J Am Chem Soc. 2003 Jul 2;125(26):7828-42. doi: 10.1021/ja028867f. J Am Chem Soc. 2003. PMID: 12823001
-
Hydroxylation of C-H bonds at carboxylate-bridged diiron centres.Philos Trans A Math Phys Eng Sci. 2005 Apr 15;363(1829):861-77; discussion 1035-40. doi: 10.1098/rsta.2004.1532. Philos Trans A Math Phys Eng Sci. 2005. PMID: 15901540 Review.
Cited by
-
Substrate-Triggered μ-Peroxodiiron(III) Intermediate in the 4-Chloro-l-Lysine-Fragmenting Heme-Oxygenase-like Diiron Oxidase (HDO) BesC: Substrate Dissociation from, and C4 Targeting by, the Intermediate.Biochemistry. 2022 Apr 19;61(8):689-702. doi: 10.1021/acs.biochem.1c00774. Epub 2022 Apr 5. Biochemistry. 2022. PMID: 35380785 Free PMC article.
-
Unprecedented (μ-1,1-Peroxo)diferric Structure for the Ambiphilic Orange Peroxo Intermediate of the Nonheme N-Oxygenase CmlI.J Am Chem Soc. 2017 Aug 2;139(30):10472-10485. doi: 10.1021/jacs.7b05389. Epub 2017 Jul 19. J Am Chem Soc. 2017. PMID: 28673082 Free PMC article.
-
Intramolecular Hydrogen-Bond Interactions Tune Reactivity in Biomimetic Bis(μ-hydroxo)dicobalt Complexes.Inorg Chem. 2021 Oct 18;60(20):15599-15609. doi: 10.1021/acs.inorgchem.1c02210. Epub 2021 Oct 4. Inorg Chem. 2021. PMID: 34606250 Free PMC article.
-
Biosynthesis and Mechanism of Action of the Cell Wall Targeting Antibiotic Hypeptin.Angew Chem Int Ed Engl. 2021 Jun 7;60(24):13579-13586. doi: 10.1002/anie.202102224. Epub 2021 May 7. Angew Chem Int Ed Engl. 2021. PMID: 33768646 Free PMC article.
-
Pulsed Multifrequency Electron Paramagnetic Resonance Spectroscopy Reveals Key Branch Points for One- vs Two-Electron Reactivity in Mn/Fe Proteins.J Am Chem Soc. 2022 Jul 13;144(27):11991-12006. doi: 10.1021/jacs.1c13738. Epub 2022 Jul 5. J Am Chem Soc. 2022. PMID: 35786920 Free PMC article.
References
-
- He J, Yao X, Vining LC. Cloning of the chloramphenicol biosynthesis genes of Streptomyces venezuelae-5230. Shenyang Yaoke Daxue Xuebao. 2006;23:731–734.
-
- Vining LC, Stuttard C. Chloramphenicol. In: Vining LC, Stuttard C, editors. Biotechnology Series. Butterworth-Heinemann; Boston: 1995. pp. 505–530. - PubMed
-
- Pacholec M, Sello JK, Walsh CT, Thomas MG. Formation of an aminoacyl-S-enzyme intermediate is a key step in the biosynthesis of chloramphenicol. Org. Biomol. Chem. 2007;5:1692–1694. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

