Altered Satellite Cell Responsiveness and Denervation Implicated in Progression of Rotator-Cuff Injury
- PMID: 27668864
- PMCID: PMC5036792
- DOI: 10.1371/journal.pone.0162494
Altered Satellite Cell Responsiveness and Denervation Implicated in Progression of Rotator-Cuff Injury
Abstract
Background: Rotator-cuff injury (RCI) is common and painful; even after surgery, joint stability and function may not recover. Relative contributions to atrophy from disuse, fibrosis, denervation, and satellite-cell responsiveness to activating stimuli are not known.
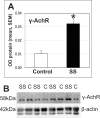
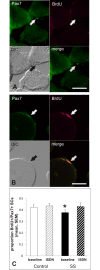
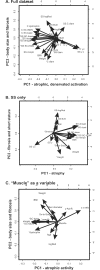
Methods and findings: Potential contributions of denervation and disrupted satellite cell responses to growth signals were examined in supraspinatus (SS) and control (ipsilateral deltoid) muscles biopsied from participants with RCI (N = 27). Biopsies were prepared for explant culture (to study satellite cell activity), immunostained to localize Pax7, BrdU, and Semaphorin 3A in satellite cells, sectioning to study blood vessel density, and western blotting to measure the fetal (γ) subunit of acetylcholine receptor (γ-AchR). Principal component analysis (PCA) for 35 parameters extracted components identified variables that contributed most to variability in the dataset. γ-AchR was higher in SS than control, indicating denervation. Satellite cells in SS had a low baseline level of activity (Pax7+ cells labelled in S-phase) versus control; only satellite cells in SS showed increased proliferative activity after nitric oxide-donor treatment. Interestingly, satellite cell localization of Semaphorin 3A, a neuro-chemorepellent, was greater in SS (consistent with fiber denervation) than control muscle at baseline. PCAs extracted components including fiber atrophy, satellite cell activity, fibrosis, atrogin-1, smoking status, vascular density, γAchR, and the time between symptoms and surgery. Use of deltoid as a control for SS was supported by PCA findings since "muscle" was not extracted as a variable in the first two principal components. SS muscle in RCI is therefore atrophic, denervated, and fibrotic, and has satellite cells that respond to activating stimuli.
Conclusions: Since SS satellite cells can be activated in culture, a NO-donor drug combined with stretching could promote muscle growth and improve functional outcome after RCI. PCAs suggest indices including satellite cell responsiveness, atrogin-1, atrophy, and innervation may predict surgical outcome.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Altered satellite cell dynamics accompany skeletal muscle atrophy during chronic illness, disuse, and aging.Curr Opin Clin Nutr Metab Care. 2017 Nov;20(6):447-452. doi: 10.1097/MCO.0000000000000409. Curr Opin Clin Nutr Metab Care. 2017. PMID: 28795971 Free PMC article. Review.
-
Atrophy, inducible satellite cell activation, and possible denervation of supraspinatus muscle in injured human rotator-cuff muscle.Am J Physiol Cell Physiol. 2015 Sep 15;309(6):C383-91. doi: 10.1152/ajpcell.00143.2015. Epub 2015 Jul 1. Am J Physiol Cell Physiol. 2015. PMID: 26135801
-
Fibrosis, low vascularity, and fewer slow fibers after rotator-cuff injury.Muscle Nerve. 2017 May;55(5):715-726. doi: 10.1002/mus.25388. Epub 2017 Jan 11. Muscle Nerve. 2017. PMID: 27571286
-
[Pax7 and depletion of satellite cell pool in prolonged denervated skeletal muscles of adult rats].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009 Jan;23(1):92-6. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009. PMID: 19192888 Chinese.
-
Skeletal muscle injury and repair: the effect of disuse and denervation on muscle and clinical relevance in pedicled and free muscle flaps.J Reconstr Microsurg. 2012 Nov;28(9):581-7. doi: 10.1055/s-0032-1315784. Epub 2012 Jun 18. J Reconstr Microsurg. 2012. PMID: 22711205 Review.
Cited by
-
Key concepts in muscle regeneration: muscle "cellular ecology" integrates a gestalt of cellular cross-talk, motility, and activity to remodel structure and restore function.Eur J Appl Physiol. 2022 Feb;122(2):273-300. doi: 10.1007/s00421-021-04865-4. Epub 2021 Dec 20. Eur J Appl Physiol. 2022. PMID: 34928395 Free PMC article. Review.
-
The Rotator Cuff Organ: Integrating Developmental Biology, Tissue Engineering, and Surgical Considerations to Treat Chronic Massive Rotator Cuff Tears.Tissue Eng Part B Rev. 2017 Aug;23(4):318-335. doi: 10.1089/ten.TEB.2016.0446. Epub 2017 Feb 9. Tissue Eng Part B Rev. 2017. PMID: 28084902 Free PMC article.
-
A focused review of myokines as a potential contributor to muscle hypertrophy from resistance-based exercise.Eur J Appl Physiol. 2020 May;120(5):941-959. doi: 10.1007/s00421-020-04337-1. Epub 2020 Mar 6. Eur J Appl Physiol. 2020. PMID: 32144492 Review.
-
Bioinformatics analysis of differentially expressed genes in rotator cuff tear patients using microarray data.J Orthop Surg Res. 2018 Nov 13;13(1):284. doi: 10.1186/s13018-018-0989-5. J Orthop Surg Res. 2018. PMID: 30424787 Free PMC article.
-
Altered satellite cell dynamics accompany skeletal muscle atrophy during chronic illness, disuse, and aging.Curr Opin Clin Nutr Metab Care. 2017 Nov;20(6):447-452. doi: 10.1097/MCO.0000000000000409. Curr Opin Clin Nutr Metab Care. 2017. PMID: 28795971 Free PMC article. Review.
References
-
- Yamaguchi K, Ditsios K, Middleton WD, Hildebolt CF, Galatz LM, Teefey SA. The demographic and morphological features of rotator cuff disease. A comparison of asymptomatic and symptomatic shoulders. JBone Joint SurgAm. 2006;88(8):1699–704. - PubMed
-
- Guckel C, Nidecker A. Diagnosis of tears in rotator-cuff-injuries. EurJRadiol. 1997;25(3):168–76. - PubMed
-
- Lee HJ, Kim YS, Ok JH, Song HJ. Apoptosis occurs throughout the diseased rotator cuff. AmJSports Med. 2013;41(10):2249–55. - PubMed
-
- Longo UG, Berton A, Khan WS, Maffulli N, Denaro V. Histopathology of rotator cuff tears. Sports MedArthrosc. 2011;19(3):227–36. - PubMed
-
- Matava MJ, Purcell DB, Rudzki JR. Partial-thickness rotator cuff tears. AmJSports Med. 2005;33(9):1405–17. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

