Prenatal Dexamethasone and Postnatal High-Fat Diet Decrease Interferon Gamma Production through an Age-Dependent Histone Modification in Male Sprague-Dawley Rats
- PMID: 27669212
- PMCID: PMC5085643
- DOI: 10.3390/ijms17101610
Prenatal Dexamethasone and Postnatal High-Fat Diet Decrease Interferon Gamma Production through an Age-Dependent Histone Modification in Male Sprague-Dawley Rats
Abstract
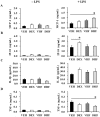
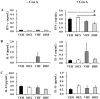
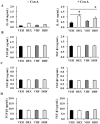
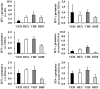
Overexposure to prenatal glucocorticoid (GC) disturbs hypothalamic-pituitary-adrenocortical axis-associated neuroendocrine metabolism and susceptibility to metabolic syndrome. A high-fat (HF) diet is a major environmental factor that can cause metabolic syndrome. We aimed to investigate whether prenatal GC plus a postnatal HF diet could alter immune programming in rat offspring. Pregnant Sprague-Dawley rats were given intraperitoneal injections of dexamethasone or saline at 14-21 days of gestation. Male offspring were then divided into four groups: vehicle, prenatal dexamethasone exposure, postnatal HF diet (VHF), and prenatal dexamethasone exposure plus a postnatal HF diet (DHF). The rats were sacrificed and adaptive immune function was evaluated. Compared to the vehicle, the DHF group had lower interferon gamma (IFN-γ) production by splenocytes at postnatal day 120. Decreases in H3K9 acetylation and H3K36me3 levels at the IFN-γ promoter correlated with decreased IFN-γ production. The impaired IFN-γ production and aberrant site-specific histone modification at the IFN-γ promoter by prenatal dexamethasone treatment plus a postnatal HF diet resulted in resilience at postnatal day 180. Prenatal dexamethasone and a postnatal HF diet decreased IFN-γ production through a site-specific and an age-dependent histone modification. These findings suggest a mechanism by which prenatal exposure to GC and a postnatal environment exert effects on fetal immunity programming.
Keywords: IFN-γ; age-dependent; high-fat diet; histone modification; prenatal glucocorticoid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Programming Effects of Prenatal Glucocorticoid Exposure with a Postnatal High-Fat Diet in Diabetes Mellitus.Int J Mol Sci. 2016 Apr 8;17(4):533. doi: 10.3390/ijms17040533. Int J Mol Sci. 2016. PMID: 27070590 Free PMC article.
-
Postnatal High-Fat Diet Increases Liver Steatosis and Apoptosis Threatened by Prenatal Dexamethasone through the Oxidative Effect.Int J Mol Sci. 2016 Mar 11;17(3):369. doi: 10.3390/ijms17030369. Int J Mol Sci. 2016. PMID: 26978357 Free PMC article.
-
Obesity programmed by prenatal dexamethasone and postnatal high-fat diet leads to distinct alterations in nutrition sensory signals and circadian-clock genes in visceral adipose tissue.Lipids Health Dis. 2019 Jan 18;18(1):19. doi: 10.1186/s12944-019-0963-1. Lipids Health Dis. 2019. PMID: 30658634 Free PMC article.
-
Prenatal dexamethasone exposure in rats results in long-term epigenetic histone modifications and tumour necrosis factor-α production decrease.Immunology. 2014 Dec;143(4):651-60. doi: 10.1111/imm.12346. Immunology. 2014. PMID: 24962734 Free PMC article.
-
Age-Dependent Effects of Prenatal Dexamethasone Exposure on Immune Responses in Male Rats.Tohoku J Exp Med. 2017 Mar;241(3):225-237. doi: 10.1620/tjem.241.225. Tohoku J Exp Med. 2017. PMID: 28344212
Cited by
-
Targeting viperin improves diet-induced glucose intolerance but not adipose tissue inflammation.Oncotarget. 2017 Sep 8;8(60):101418-101436. doi: 10.18632/oncotarget.20724. eCollection 2017 Nov 24. Oncotarget. 2017. PMID: 29254175 Free PMC article.
-
Prenatal dexamethasone and postnatal high-fat diet have a synergistic effect of elevating blood pressure through a distinct programming mechanism of systemic and adipose renin-angiotensin systems.Lipids Health Dis. 2018 Mar 14;17(1):50. doi: 10.1186/s12944-018-0701-0. Lipids Health Dis. 2018. PMID: 29540174 Free PMC article.
-
Prenatal dexamethasone exposure reduces osteoprogenitor proliferation in mice via histone modifications at the Mkp-1 gene locus.Commun Biol. 2024 Nov 28;7(1):1589. doi: 10.1038/s42003-024-07288-x. Commun Biol. 2024. PMID: 39609620 Free PMC article.
-
The Functional DNA Methylation Signatures Relevant to Altered Immune Response of Neonatal T Cells with l-Arginine Supplementation.Nutrients. 2021 Aug 13;13(8):2780. doi: 10.3390/nu13082780. Nutrients. 2021. PMID: 34444938 Free PMC article.
-
Maternal Metformin Treatment Reprograms Maternal High-Fat Diet-Induced Hepatic Steatosis in Offspring Associated with Placental Glucose Transporter Modifications.Int J Mol Sci. 2022 Nov 17;23(22):14239. doi: 10.3390/ijms232214239. Int J Mol Sci. 2022. PMID: 36430717 Free PMC article.
References
-
- Brownfoot F.C., Gagliardi D.I., Bain E., Middleton P., Crowther C.A. Different corticosteroids and regimens for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 2013;8:CD006764. - PubMed
-
- Drake A.J., Raubenheimer P.J., Kerrigan D., McInnes K.J., Seckl J.R., Walker B.R. Prenatal dexamethasone programs expression of genes in liver and adipose tissue and increased hepatic lipid accumulation but not obesity on a high-fat diet. Endocrinology. 2010;151:1581–1587. doi: 10.1210/en.2009-1088. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

