Sensitivity Analysis of the NPM-ALK Signalling Network Reveals Important Pathways for Anaplastic Large Cell Lymphoma Combination Therapy
- PMID: 27669408
- PMCID: PMC5036789
- DOI: 10.1371/journal.pone.0163011
Sensitivity Analysis of the NPM-ALK Signalling Network Reveals Important Pathways for Anaplastic Large Cell Lymphoma Combination Therapy
Abstract
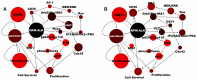
A large subset of anaplastic large cell lymphoma (ALCL) patients harbour a somatic aberration in which anaplastic lymphoma kinase (ALK) is fused to nucleophosmin (NPM) resulting in a constitutively active signalling fusion protein, NPM-ALK. We computationally simulated the signalling network which mediates pathological cell survival and proliferation through NPM-ALK to identify therapeutically targetable nodes through which it may be possible to regain control of the tumourigenic process. The simulations reveal the predominant role of the VAV1-CDC42 (cell division control protein 42) pathway in NPM-ALK-driven cellular proliferation and of the Ras / mitogen-activated ERK kinase (MEK) / extracellular signal-regulated kinase (ERK) cascade in controlling cell survival. Our results also highlight the importance of a group of interleukins together with the Janus kinase 3 (JAK3) / signal transducer and activator of transcription 3 (STAT3) signalling in the development of NPM-ALK derived ALCL. Depending on the activity of JAK3 and STAT3, the system may also be sensitive to activation of protein tyrosine phosphatase-1 (SHP1), which has an inhibitory effect on cell survival and proliferation. The identification of signalling pathways active in tumourigenic processes is of fundamental importance for effective therapies. The prediction of alternative pathways that circumvent classical therapeutic targets opens the way to preventive approaches for countering the emergence of cancer resistance.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Aisner DL, Nguyen TT, Paskulin DD, Le AT, Haney J, Schulte N, et al. ROS1 and ALK fusions in colorectal cancer, with evidence of intratumoral heterogeneity for molecular drivers. Mol Cancer Res. 2014. January;12(1):111–118. [PubMed Central:PMC4140177]. 10.1158/1541-7786.MCR-13-0479-T - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

