Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells
- PMID: 27669441
- PMCID: PMC5366272
- DOI: 10.1038/onc.2016.353
Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells
Abstract
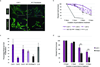
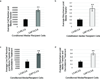
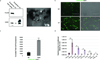
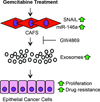
Cancer-associated fibroblasts (CAFs) comprise the majority of the tumor bulk of pancreatic ductal adenocarcinomas (PDACs). Current efforts to eradicate these tumors focus predominantly on targeting the proliferation of rapidly growing cancer epithelial cells. We know that this is largely ineffective with resistance arising in most tumors following exposure to chemotherapy. Despite the long-standing recognition of the prominence of CAFs in PDAC, the effect of chemotherapy on CAFs and how they may contribute to drug resistance in neighboring cancer cells is not well characterized. Here, we show that CAFs exposed to chemotherapy have an active role in regulating the survival and proliferation of cancer cells. We found that CAFs are intrinsically resistant to gemcitabine, the chemotherapeutic standard of care for PDAC. Further, CAFs exposed to gemcitabine significantly increase the release of extracellular vesicles called exosomes. These exosomes increased chemoresistance-inducing factor, Snail, in recipient epithelial cells and promote proliferation and drug resistance. Finally, treatment of gemcitabine-exposed CAFs with an inhibitor of exosome release, GW4869, significantly reduces survival in co-cultured epithelial cells, signifying an important role of CAF exosomes in chemotherapeutic drug resistance. Collectively, these findings show the potential for exosome inhibitors as treatment options alongside chemotherapy for overcoming PDAC chemoresistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

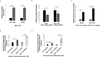

Comment in
-
Pancreatic fibroblast exosomes regulate survival of cancer cells.Oncogene. 2017 Jun 22;36(25):3648-3649. doi: 10.1038/onc.2016.528. Epub 2017 Feb 6. Oncogene. 2017. PMID: 28166198 No abstract available.
References
-
- Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nature reviews. 2010;7(3):163–172. - PubMed
-
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–2921. - PubMed
-
- Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2007;297(3):267–277. - PubMed
-
- Kleeff J, Beckhove P, Esposito I, Herzig S, Huber PE, Lohr JM, et al. Pancreatic cancer microenvironment. Int J Cancer. 2007;121(4):699–705. - PubMed
-
- Chu GC, Kimmelman AC, Hezel AF, DePinho RA. Stromal biology of pancreatic cancer. Journal of cellular biochemistry. 2007;101(4):887–907. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

