Mechanism and Regulation of NLRP3 Inflammasome Activation
- PMID: 27669650
- PMCID: PMC5123939
- DOI: 10.1016/j.tibs.2016.09.002
Mechanism and Regulation of NLRP3 Inflammasome Activation
Abstract
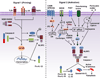
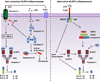
Members of the nucleotide-binding domain and leucine-rich repeat (LRR)-containing (NLR) family and the pyrin and HIN domain (PYHIN) family can form multiprotein complexes termed 'inflammasomes'. The biochemical function of inflammasomes is to activate caspase-1, which leads to the maturation of interleukin 1 beta (IL-1β) and IL-18 and the induction of pyroptosis, a form of cell death. Unlike other inflammasomes, the NLRP3 inflammasome can be activated by diverse stimuli. The importance of the NLRP3 inflammasome in immunity and human diseases has been well documented, but the mechanism and regulation of its activation remain unclear. In this review we summarize current understanding of the mechanism and regulation of NLRP3 inflammasome activation as well as recent advances in the noncanonical and alternative inflammasome pathways.
Keywords: K(+) efflux; NLRP3 inflammasome; Nek7; alternative inflammasome; noncanonical inflammasome.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures
References
-
- Martinon F, et al. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Molecular Cell. 2002;10:417–426. - PubMed
-
- Wang L, et al. PYPAF7, a novel PYRIN-containing Apaf1-like protein that regulates activation of NF-kappa B and caspase-1-dependent cytokine processing. J Biol Chem. 2002;277:29874–29880. - PubMed
-
- Dinarello CA. Immunological and Inflammatory Functions of the Interleukin-1 Family. Annual Review of Immunology. 2009:519–550. - PubMed
-
- Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8:1812–1825. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

