Regulation of Linear Ubiquitin Chain Assembly Complex by Caspase-Mediated Cleavage of RNF31
- PMID: 27669734
- PMCID: PMC5126289
- DOI: 10.1128/MCB.00474-16
Regulation of Linear Ubiquitin Chain Assembly Complex by Caspase-Mediated Cleavage of RNF31
Abstract
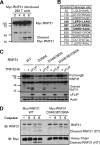
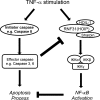
Cell death and survival signaling pathways have opposed but fundamental functions for various cellular processes and maintain cell homeostasis through cross talk. Here we report a novel mechanism of interaction between these two pathways through the cleavage of RNF31 by caspases. RNF31, a component of the linear ubiquitin chain assembly complex (LUBAC), regulates cell survival by inducing linear ubiquitination of NF-κB signaling components. We found that RNF31 is cleaved under apoptosis conditions through various stimulations. The effector caspases caspase 3 and caspase 6 are responsible for this event, and aspartates 348, 387, and 390 were identified as target sites for this cleavage. Cleavage of RNF31 suppressed its ability to activate NF-κB signaling; thus, mutation of cleavage sites inhibited the induction of apoptosis by treatment with tumor necrosis factor alpha (TNF-α). Our findings elucidate a novel regulatory loop between cell death and the survival signal and may provide guidance for the development of therapeutic strategies for cancers through the sensitization of tumor cells to death-inducing drugs.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures






References
-
- Ikeda F, Deribe YL, Skanland SS, Stieglitz B, Grabbe C, Franz-Wachtel M, van Wijk SJ, Goswami P, Nagy V, Terzic J, Tokunaga F, Androulidaki A, Nakagawa T, Pasparakis M, Iwai K, Sundberg JP, Schaefer L, Rittinger K, Macek B, Dikic I. 2011. SHARPIN forms a linear ubiquitin ligase complex regulating NF-κB activity and apoptosis. Nature 471:637–641. doi:10.1038/nature09814. - DOI - PMC - PubMed
-
- Gerlach B, Cordier SM, Schmukle AC, Emmerich CH, Rieser E, Haas TL, Webb AI, Rickard JA, Anderton H, Wong WW, Nachbur U, Gangoda L, Warnken U, Purcell AW, Silke J, Walczak H. 2011. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature 471:591–596. doi:10.1038/nature09816. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
