Regulation of the IL-10-driven macrophage phenotype under incoherent stimuli
- PMID: 27670945
- PMCID: PMC5292318
- DOI: 10.1177/1753425916668243
Regulation of the IL-10-driven macrophage phenotype under incoherent stimuli
Abstract
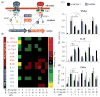
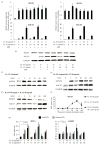
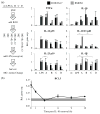
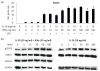
Macrophages are ubiquitous innate immune cells that play a central role in health and disease by adopting distinct phenotypes, which are broadly divided into classical inflammatory responses and alternative responses that promote immune suppression and wound healing. Although macrophages are attractive therapeutic targets, incomplete understanding of this functional choice limits clinical manipulation. While individual stimuli, pathways, and genes involved in macrophage functional responses have been identified, how macrophages evaluate complex in vivo milieus comprising multiple divergent stimuli remains poorly understood. Here, we used combinations of "incoherent" stimuli-those that individually promote distinct macrophage phenotypes-to elucidate how the immunosuppressive, IL-10-driven macrophage phenotype is induced, maintained, and modulated under such combinatorial stimuli. The IL-10-induced immunosuppressive phenotype was largely insensitive to co-administered IL-12, which has been reported to modulate macrophage phenotype, but maintaining the immunosuppressive phenotype required sustained exposure to IL-10. Our data implicate the intracellular protein, BCL3, as a key mediator of the IL-10-driven phenotype. Notably, co-administration of IFN-γ disrupted an IL-10-mediated positive feedback loop that may reinforce the immunosuppressive phenotype. This novel combinatorial perturbation approach thus generated new insights into macrophage decision making and local immune network function.
Keywords: Interleukin-10 (IL-10); macrophage polarization; macrophages; tumor-associated macrophages (TAM).
Figures

References
-
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–86. - PubMed
-
- Sica A, Larghi P, Mancino A, et al. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18:349–55. - PubMed
-
- Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750–61. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

