Antigen Targeting to Human HLA Class II Molecules Increases Efficacy of DNA Vaccination
- PMID: 27671110
- PMCID: PMC5073356
- DOI: 10.4049/jimmunol.1600893
Antigen Targeting to Human HLA Class II Molecules Increases Efficacy of DNA Vaccination
Abstract
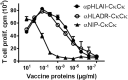
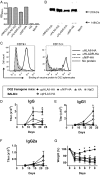
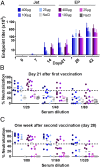
It has been difficult to translate promising results from DNA vaccination in mice to larger animals and humans. Previously, DNA vaccines encoding proteins that target Ag to MHC class II (MHC-II) molecules on APCs have been shown to induce rapid, enhanced, and long-lasting Ag-specific Ab titers in mice. In this study, we describe two novel DNA vaccines that as proteins target HLA class II (HLA-II) molecules. These vaccine proteins cross-react with MHC-II molecules in several species of larger mammals. When tested in ferrets and pigs, a single DNA delivery with low doses of the HLA-II-targeted vaccines resulted in rapid and increased Ab responses. Importantly, painless intradermal jet delivery of DNA was as effective as delivery by needle injection followed by electroporation. As an indication that the vaccines could also be useful for human application, HLA-II-targeted vaccine proteins were found to increase human CD4+ T cell responses by a factor of ×103 in vitro. Thus, targeting of Ag to MHC-II molecules may represent an attractive strategy for increasing efficacy of DNA vaccines in larger animals and humans.
Copyright © 2016 by The American Association of Immunologists, Inc.
Figures


Similar articles
-
Efficient vaccine against pandemic influenza: combining DNA vaccination and targeted delivery to MHC class II molecules.Expert Rev Vaccines. 2015 Jun;14(6):805-14. doi: 10.1586/14760584.2015.1029919. Epub 2015 Mar 27. Expert Rev Vaccines. 2015. PMID: 25818107 Review.
-
CD205 antigen targeting combined with dendritic cell recruitment factors and antigen-linked CD40L activation primes and expands significant antigen-specific antibody and CD4(+) T cell responses following DNA vaccination of outbred animals.Vaccine. 2012 Feb 21;30(9):1624-35. doi: 10.1016/j.vaccine.2011.12.110. Epub 2012 Jan 10. Vaccine. 2012. PMID: 22240344
-
CD40/APC-specific antibodies with three T-cell epitopes loaded in the constant domains induce CD4+ T-cell responses.Protein Eng Des Sel. 2012 Mar;25(3):89-96. doi: 10.1093/protein/gzr063. Epub 2012 Jan 10. Protein Eng Des Sel. 2012. PMID: 22233931
-
Targeting antigen to MHC Class I and Class II antigen presentation pathways for malaria DNA vaccines.Immunol Lett. 2007 Aug 15;111(2):92-102. doi: 10.1016/j.imlet.2007.05.007. Epub 2007 Jun 18. Immunol Lett. 2007. PMID: 17604849
-
Approaches to improved targeting of DNA vaccines.Hum Vaccin. 2011 Dec;7(12):1271-81. doi: 10.4161/hv.7.12.17983. Epub 2011 Dec 1. Hum Vaccin. 2011. PMID: 22108038 Review.
Cited by
-
The anti-influenza M2e antibody response is promoted by XCR1 targeting in pig skin.Sci Rep. 2017 Aug 9;7(1):7639. doi: 10.1038/s41598-017-07372-9. Sci Rep. 2017. PMID: 28794452 Free PMC article.
-
Recent Advances in DNA Vaccines against Lung Cancer: A Mini Review.Vaccines (Basel). 2022 Sep 21;10(10):1586. doi: 10.3390/vaccines10101586. Vaccines (Basel). 2022. PMID: 36298450 Free PMC article. Review.
-
Dendritic Cells Targeting Lactobacillus plantarum Strain NC8 with a Surface-Displayed Single-Chain Variable Fragment of CD11c Induce an Antigen-Specific Protective Cellular Immune Response.Infect Immun. 2020 Jan 22;88(2):e00759-19. doi: 10.1128/IAI.00759-19. Print 2020 Jan 22. Infect Immun. 2020. PMID: 31740528 Free PMC article.
-
Multidrug resistant Acinetobacter baumannii: A study on its pathogenesis and therapeutics.Curr Res Microb Sci. 2024 Dec 6;8:100331. doi: 10.1016/j.crmicr.2024.100331. eCollection 2025. Curr Res Microb Sci. 2024. PMID: 39802320 Free PMC article. Review.
-
A novel SARS-CoV-2 Beta RBD DNA vaccine directly targeted to antigen-presenting cells induces strong humoral and T cell responses.Sci Rep. 2023 Nov 2;13(1):18902. doi: 10.1038/s41598-023-46223-8. Sci Rep. 2023. PMID: 37919366 Free PMC article.
References
-
- Bergman P. J., Camps-Palau M. A., McKnight J. A., Leibman N. F., Craft D. M., Leung C., Liao J., Riviere I., Sadelain M., Hohenhaus A. E., et al. 2006. Development of a xenogeneic DNA vaccine program for canine malignant melanoma at the Animal Medical Center. Vaccine 24: 4582–4585. - PubMed
-
- Davis B. S., Chang G. J., Cropp B., Roehrig J. T., Martin D. A., Mitchell C. J., Bowen R., Bunning M. L. 2001. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J. Virol. 75: 4040–4047. - PMC - PubMed
-
- Kawamura H., Berzofsky J. A. 1986. Enhancement of antigenic potency in vitro and immunogenicity in vivo by coupling the antigen to anti-immunoglobulin. J. Immunol. 136: 58–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

