HIF-2α in Resting Macrophages Tempers Mitochondrial Reactive Oxygen Species To Selectively Repress MARCO-Dependent Phagocytosis
- PMID: 27671111
- PMCID: PMC5101127
- DOI: 10.4049/jimmunol.1600402
HIF-2α in Resting Macrophages Tempers Mitochondrial Reactive Oxygen Species To Selectively Repress MARCO-Dependent Phagocytosis
Abstract
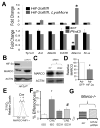
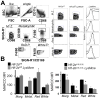
Hypoxia-inducible factor (HIF)-α isoforms regulate key macrophage (MΦ) functions during ischemic inflammation. HIF-2α drives proinflammatory cytokine production; however, the requirements for HIF-2α during other key MΦ functions, including phagocytosis, are unknown. In contrast to HIF-1α, HIF-2α was not required for hypoxic phagocytic uptake. Surprisingly, basal HIF-2α levels under nonhypoxic conditions were necessary and sufficient to suppress phagocytosis. Screening approaches revealed selective induction of the scavenger receptor MARCO, which was required for enhanced engulfment. Chromatin immunoprecipitation identified the antioxidant NRF2 as being directly responsible for inducing Marco Concordantly, Hif-2α-/- MΦs exhibited reduced antioxidant gene expression, and inhibition of mitochondrial reactive oxygen species suppressed Marco expression and phagocytic uptake. Ex vivo findings were recapitulated in vivo; the enhanced engulfment phenotype resulted in increased bacterial clearance and cytokine suppression. Importantly, natural induction of Hif-2α by IL-4 also suppressed MARCO-dependent phagocytosis. Thus, unlike most characterized prophagocytic regulators, HIF-2α can act as a phagocytic repressor. Interestingly, this occurs in resting MΦs through tempering of steady-state mitochondrial reactive oxygen species. In turn, HIF-2α promotes MΦ quiescence by blocking a MARCO bacterial-response pathway. IL-4 also drives HIF-2α suppression of MARCO, leading to compromised bacterial immunosurveillance in vivo.
Copyright © 2016 by The American Association of Immunologists, Inc.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

