Devitalisation of human cartilage by high hydrostatic pressure treatment: Subsequent cultivation of chondrocytes and mesenchymal stem cells on the devitalised tissue
- PMID: 27671122
- PMCID: PMC5037397
- DOI: 10.1038/srep33747
Devitalisation of human cartilage by high hydrostatic pressure treatment: Subsequent cultivation of chondrocytes and mesenchymal stem cells on the devitalised tissue
Abstract
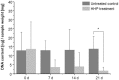
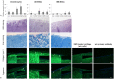
The regeneration of cartilage lesions still represents a major challenge. Cartilage has a tissue-specific architecture, complicating recreation by synthetic biomaterials. A novel approach for reconstruction is the use of devitalised cartilage. Treatment with high hydrostatic pressure (HHP) achieves devitalisation while biomechanical properties are remained. Therefore, in the present study, cartilage was devitalised using HHP treatment and the potential for revitalisation with chondrocytes and mesenchymal stem cells (MSCs) was investigated. The devitalisation of cartilage was performed by application of 480 MPa over 10 minutes. Effective cellular inactivation was demonstrated by the trypan blue exclusion test and DNA quantification. Histology and electron microscopy examinations showed undamaged cartilage structure after HHP treatment. For revitalisation chondrocytes and MSCs were cultured on devitalised cartilage without supplementation of chondrogenic growth factors. Both chondrocytes and MSCs significantly increased expression of cartilage-specific genes. ECM stainings showed neocartilage-like structure with positive AZAN staining as well as collagen type II and aggrecan deposition after three weeks of cultivation. Our results showed that HHP treatment caused devitalisation of cartilage tissue. ECM proteins were not influenced, thus, providing a scaffold for chondrogenic differentiation of MSCs and chondrocytes. Therefore, using HHP-treated tissue might be a promising approach for cartilage repair.
Figures




References
-
- Davies-Tuck M. L. et al. The natural history of cartilage defects in people with knee osteoarthritis. Osteoarthritis and Cartilage. 16, 337–342 (2008). - PubMed
-
- Abazari A., Jomha N. M., Elliott J. A. W. & McGann L. E. Cryopreservation of articular cartilage. Cryobiology. 2013, 201–209 (2013). - PubMed
-
- Hindle P., Hendry J. L., Keating J. F. & Biant L. C. Autologous osteochondral mosaicplasty or TruFit plugs for cartilage repair. Knee Surg Sports Traumatol Arthrosc. 22, 1235–1240 (2014). - PubMed
-
- Redman S. N., Oldfield S. F. & Archer C. W. Current strategies for articular cartilage repair. Eur Cell Mater. 9, 23–32 (2005). - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

