AXL Inhibition Suppresses the DNA Damage Response and Sensitizes Cells to PARP Inhibition in Multiple Cancers
- PMID: 27671334
- PMCID: PMC5215967
- DOI: 10.1158/1541-7786.MCR-16-0157
AXL Inhibition Suppresses the DNA Damage Response and Sensitizes Cells to PARP Inhibition in Multiple Cancers
Abstract
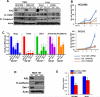
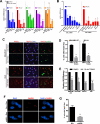
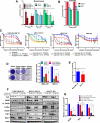
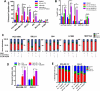
Epithelial to mesenchymal transition (EMT) is associated with a wide range of changes in cancer cells, including stemness, chemo- and radio-resistance, and metastasis. The mechanistic role of upstream mediators of EMT has not yet been well characterized. Recently, we showed that non-small cell lung cancers (NSCLC) that have undergone EMT overexpress AXL, a receptor tyrosine kinase. AXL is also overexpressed in a subset of triple-negative breast cancers (TNBC) and head and neck squamous cell carcinomas (HNSCC), and its overexpression has been associated with more aggressive tumor behavior and linked to resistance to chemotherapy, radiotherapy, and targeted therapy. Because the DNA repair pathway is also altered in patient tumor specimens overexpressing AXL, it is hypothesized that modulation of AXL in cells that have undergone EMT will sensitize them to agents targeting the DNA repair pathway. Downregulation or inhibition of AXL directly reversed the EMT phenotype, led to decreased expression of DNA repair genes, and diminished efficiency of homologous recombination (HR) and RAD51 foci formation. As a result, AXL inhibition caused a state of HR deficiency in the cells, making them sensitive to inhibition of the DNA repair protein, PARP1. AXL inhibition synergized with PARP inhibition, leading to apoptotic cell death. AXL expression also associated positively with markers of DNA repair across TNBC, HNSCC, and NSCLC patient cohorts.
Implications: The novel role for AXL in DNA repair, linking it to EMT, suggests that AXL can be an effective therapeutic target in combination with targeted therapy such as PARP inhibitors in several different malignancies. Mol Cancer Res; 15(1); 45-58. ©2016 AACR.
©2016 American Association for Cancer Research.
Figures

References
-
- Zhang YX, Knyazev PG, Cheburkin YV, Sharma K, Knyazev YP, Orfi L, et al. AXL is a potential target for therapeutic intervention in breast cancer progression. Cancer research. 2008;68:1905–15. - PubMed
-
- Li Y, Ye X, Tan C, Hongo JA, Zha J, Liu J, et al. Axl as a potential therapeutic target in cancer: role of Axl in tumor growth, metastasis and angiogenesis. Oncogene. 2009;28:3442–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

