Tyrosine phosphorylation stimulates activity of human RAD51 recombinase through altered nucleoprotein filament dynamics
- PMID: 27671650
- PMCID: PMC5068273
- DOI: 10.1073/pnas.1604807113
Tyrosine phosphorylation stimulates activity of human RAD51 recombinase through altered nucleoprotein filament dynamics
Abstract
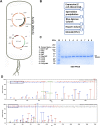
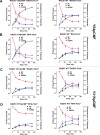
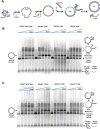
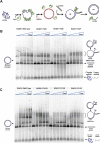
The DNA strand exchange protein RAD51 facilitates the central step in homologous recombination, a process fundamentally important for accurate repair of damaged chromosomes, restart of collapsed replication forks, and telomere maintenance. The active form of RAD51 is a nucleoprotein filament that assembles on single-stranded DNA (ssDNA) at the sites of DNA damage. The c-Abl tyrosine kinase and its oncogenic counterpart BCR-ABL fusion kinase phosphorylate human RAD51 on tyrosine residues 54 and 315. We combined biochemical reconstitutions of the DNA strand exchange reactions with total internal reflection fluorescence microscopy to determine how the two phosphorylation events affect the biochemical activities of human RAD51 and properties of the RAD51 nucleoprotein filament. By mimicking RAD51 tyrosine phosphorylation with a nonnatural amino acid, p-carboxymethyl-l-phenylalanine (pCMF), we demonstrated that Y54 phosphorylation enhances the RAD51 recombinase activity by at least two different mechanisms, modifies the RAD51 nucleoprotein filament formation, and allows RAD51 to compete efficiently with ssDNA binding protein RPA. In contrast, Y315 phosphorylation has little effect on the RAD51 activities. Based on our work and previous cellular studies, we propose a mechanism underlying RAD51 activation by c-Abl/BCR-ABL kinases.
Keywords: RAD51 recombinase; c-Abl tyrosine kinase; homologous recombination; phosphorylation; single-molecule total internal reflection fluorescence microscopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Observation and Analysis of RAD51 Nucleation Dynamics at Single-Monomer Resolution.Methods Enzymol. 2018;600:201-232. doi: 10.1016/bs.mie.2017.12.008. Epub 2018 Feb 1. Methods Enzymol. 2018. PMID: 29458759 Free PMC article.
-
Tyrosine phosphorylation enhances RAD52-mediated annealing by modulating its DNA binding.EMBO J. 2011 Jul 29;30(16):3368-82. doi: 10.1038/emboj.2011.238. EMBO J. 2011. PMID: 21804533 Free PMC article.
-
Detection of c-Abl kinase-promoted phosphorylation of Rad51 by specific antibodies reveals that Y54 phosphorylation is dependent on that of Y315.FEBS Lett. 2009 Jun 18;583(12):1867-72. doi: 10.1016/j.febslet.2009.04.044. Epub 2009 May 7. FEBS Lett. 2009. PMID: 19427856
-
Insights into the control of RAD51 nucleoprotein filament dynamics from single-molecule studies.Curr Opin Genet Dev. 2021 Dec;71:182-187. doi: 10.1016/j.gde.2021.09.001. Epub 2021 Sep 24. Curr Opin Genet Dev. 2021. PMID: 34571340 Review.
-
Single-molecule imaging brings Rad51 nucleoprotein filaments into focus.Trends Cell Biol. 2010 May;20(5):269-76. doi: 10.1016/j.tcb.2010.02.004. Epub 2010 Mar 17. Trends Cell Biol. 2010. PMID: 20299221 Free PMC article. Review.
Cited by
-
Mechanism of BCDX2-mediated RAD51 nucleation on short ssDNA stretches and fork DNA.Nucleic Acids Res. 2024 Oct 28;52(19):11738-11752. doi: 10.1093/nar/gkae770. Nucleic Acids Res. 2024. PMID: 39268578 Free PMC article.
-
The protein phosphatase EYA4 promotes homologous recombination (HR) through dephosphorylation of tyrosine 315 on RAD51.Nucleic Acids Res. 2024 Feb 9;52(3):1173-1187. doi: 10.1093/nar/gkad1177. Nucleic Acids Res. 2024. PMID: 38084915 Free PMC article.
-
RAD51 Gene Family Structure and Function.Annu Rev Genet. 2020 Nov 23;54:25-46. doi: 10.1146/annurev-genet-021920-092410. Epub 2020 Jul 14. Annu Rev Genet. 2020. PMID: 32663049 Free PMC article. Review.
-
Crosstalk between CST and RPA regulates RAD51 activity during replication stress.Nat Commun. 2021 Nov 5;12(1):6412. doi: 10.1038/s41467-021-26624-x. Nat Commun. 2021. PMID: 34741010 Free PMC article.
-
RAD52 prevents accumulation of -dependent replication gaps at perturbed replication forks in human cells.bioRxiv [Preprint]. 2024 Aug 17:2023.04.12.536536. doi: 10.1101/2023.04.12.536536. bioRxiv. 2024. PMID: 37090680 Free PMC article. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

