Multiplicity of morphologies in poly (l-lactide) bioresorbable vascular scaffolds
- PMID: 27671659
- PMCID: PMC5081625
- DOI: 10.1073/pnas.1602311113
Multiplicity of morphologies in poly (l-lactide) bioresorbable vascular scaffolds
Abstract
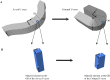
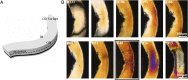
Poly(l-lactide) (PLLA) is the structural material of the first clinically approved bioresorbable vascular scaffold (BVS), a promising alternative to permanent metal stents for treatment of coronary heart disease. BVSs are transient implants that support the occluded artery for 6 mo and are completely resorbed in 2 y. Clinical trials of BVSs report restoration of arterial vasomotion and elimination of serious complications such as late stent thrombosis. It is remarkable that a scaffold made from PLLA, known as a brittle polymer, does not fracture when crimped onto a balloon catheter or during deployment in the artery. We used X-ray microdiffraction to discover how PLLA acquired ductile character and found that the crimping process creates localized regions of extreme anisotropy; PLLA chains in the scaffold change orientation from the hoop direction to the radial direction on micrometer-scale distances. This multiplicity of morphologies in the crimped scaffold works in tandem to enable a low-stress response during deployment, which avoids fracture of the PLLA hoops and leaves them with the strength needed to support the artery. Thus, the transformations of the semicrystalline PLLA microstructure during crimping explain the unexpected strength and ductility of the current BVS and point the way to thinner resorbable scaffolds in the future.
Keywords: coronary heart disease; ductility; microdiffraction; poly (l-lactide); structural transformation.
Conflict of interest statement
M.B.K. and J.P.O. are employees of Abbott Vascular. Funding for this research was provided by Abbott Vascular.
Figures














References
-
- Nichols M, Townsend N, Scarborough P, Rayner M. Cardiovascular disease in Europe 2014: Epidemiological update. Eur Heart J. 2014;35(42):2950–2959. - PubMed
-
- Ormiston JA, et al. A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB): A prospective open-label trial. Lancet. 2008;371(9616):899–907. - PubMed
-
- Wiebe J, Nef HM, Hamm CW. Current status of bioresorbable scaffolds in the treatment of coronary artery disease. J Am Coll Cardiol. 2014;64(23):2541–2551. - PubMed
-
- Stettler C, et al. Outcomes associated with drug-eluting and bare-metal stents: A collaborative network meta-analysis. Lancet. 2007;370(9591):937–948. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

