Three-dimensional microCT imaging of mouse development from early post-implantation to early postnatal stages
- PMID: 27671873
- PMCID: PMC5405732
- DOI: 10.1016/j.ydbio.2016.09.011
Three-dimensional microCT imaging of mouse development from early post-implantation to early postnatal stages
Abstract
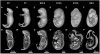
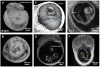
In this work, we report the use of iodine-contrast microCT to perform high-throughput 3D morphological analysis of mouse embryos and neonates between embryonic day 8.5 to postnatal day 3, with high spatial resolution up to 3µm/voxel. We show that mouse embryos at early stages can be imaged either within extra embryonic tissues such as the yolk sac or the decidua without physically disturbing the embryos. This method enables a full, undisturbed analysis of embryo turning, allantois development, vitelline vessels remodeling, yolk sac and early placenta development, which provides increased insights into early embryonic lethality in mutant lines. Moreover, these methods are inexpensive, simple to learn and do not require substantial processing time, making them ideal for high throughput analysis of mouse mutants with embryonic and early postnatal lethality.
Keywords: Embryonic lethal screening; IMPC; MicroCT.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Adams D, Baldock R, Bhattacharya S, Copp AJ, Dickinson M, Greene NDE, Henkelman M, Justice M, Mohun T, Murray SA, Pauws E, Raess M, Rossant J, Weaver T, West D. Bloomsbury report on mouse embryo phenotyping: recommendations from the IMPC workshop on embryonic lethal screening. Dis Models Mech. 2013;6:571–579. http://dx.doi.org/10.1242/dmm.011833. - DOI - PMC - PubMed
-
- Brown JM, Horner NR, Lawson TN, Fiegel T, Greenway S, Morgan H, Ring N, Santos L, Sneddon D, Teboul L, Vibert J, Yaikhom G, Westerberg H, Mallon AM. A bioimage informatics platform for high-throughput embryo phenotyping. Brief Bioinformatics. 2016 http://dx.doi.org/10.1093/bib/bbw101. - DOI - PMC - PubMed
-
- Degenhardt K, Wright AC, Horng D, Padmanabhan A, Epstein JA. Rapid 3D phenotyping of cardiovascular development in mouse embryos by micro-CT with iodine staining. Circ Cardiovasc Imaging. 2010;3:314–322. http://dx.doi.org/10.1161/CIRCIMAGING.109.918482. - DOI - PMC - PubMed
-
- Dickinson ME, Flenniken AM, Ji X, Teboul L, Wong MD, White JK, Meehan TF, Weninger WJ, Westerberg H, Adissu H, Baker CN, Bower L, Brown JM, Caddle LB, Chiani F, Clary D, Cleak J, Daly MJ, Denegre JM, Doe B, Dolan ME, Edie SM, Fuchs H, Gailus-Durner V, Galli A, Gambadoro A, Gallegos J, Guo S, Horner NR, Hsu CW, Johnson SJ, Kalaga S, Keith LC, Lanoue L, Lawson TN, Lek M, Mark M, Marschall S, Mason J, McElwee ML, Newbigging S, Nutter LMJ, Peterson KA, Ramirez-Solis R, Rowland DJ, Ryder E, Samocha KE, Seavitt JR, Selloum M, Szoke-Kovacs Z, Tamura M, Trainor AG, Tudose I, Wakana S, Warren J, Wendling O, West DB, Wong L, Yoshiki A, MacArthur DG, Tocchini-Valentini GP, Gao X, Flicek P, Bradley A, Skarnes WC, Justice MJ, Parkinson HE, Moore M, Wells S, Braun RE, Svenson KL, de Angelis MH, Herault Y, Mohun T, Mallon AM, Henkelman RM, Brown SDM, Adams DJ, Lloyd KCK, McKerlie C, Beaudet AL, Bućan M, Murray SA International Mouse Phenotyping Consortium, Jackson Laboratory, Infrastructure Nationale PHENOMIN, Institut Clinique de la Souris (ICS), Charles River Laboratories, MRC Harwell, Toronto Centre for Phenogenomics, Wellcome Trust Sanger Institute, RIKEN BioResource Center. High-throughput discovery of novel developmental phenotypes. Nature. 2016 doi: 10.1038/nature19356. - DOI - PubMed
-
- Feldkamp LA, Davis LC, Kress JW. Practical cone-beam algorithm. J Opt Soc Am A. 1984;1:612–619. http://dx.doi.org/10.1364/JOSAA.1.000612. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

