p75 neurotrophin receptor regulates differential mineralization of rat ectomesenchymal stem cells
- PMID: 27672006
- PMCID: PMC6529075
- DOI: 10.1111/cpr.12290
p75 neurotrophin receptor regulates differential mineralization of rat ectomesenchymal stem cells
Abstract
Objectives: The aim of this study was to investigate whether p75NTR (p75 neurotrophin receptor) regulates differential mineralization capacity of rEMSCs (rat ectomesenchymal stem cells) and underlying mechanisms associated with Mage-D1 (melanoma-associated antigens-D1).
Materials and methods: Immunohistochemical staining of p75NTR in developing tooth germs was performed on E12.5d (embryonic 12.5 days) and E19.5d (embryonic 19.5 days). E12.5d EMSCs and E19.5d EMSCs were isolated in the same pregnant Sprague-Dawley rats from embryonic maxillofacial processes and tooth germs. p75NTR small-interfering RNA, p75NTR overexpression plasmid, Mage-D1 small-interfering RNA and recombined rat NGF were used to transfect cells.
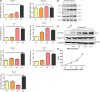
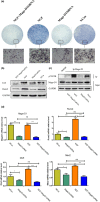
Results: p75NTR was expressed in epithelial-mesenchymal interaction areas at E12.5d and E19.5d tooth germ development stages. E19.5d EMSCs had higher p75NTR expression levels and differential mineralization capacity but lower levels of cell proliferation. Under induction by mineralized culture medium, the potential of differential mineralization had identical trends in regulation of p75NTR in EMSCs; Mage-D1 did not fluctuate and TrkA was not expressed. Binding of p75NTR and Mage-D1 were detected. Mage-D1 knockdown significantly down-regulated expression of related genes, which NGF could not rescue.
Conclusion: p75NTR participated in tooth germ development stages and mediated differential mineralization of EMSCs. p75NTR played a critical role in regulating the potential of differential mineralization of EMSCs. Mage-D1 seemed to act as a bridge in the underlying mechanism of effects of p75NTR.
© 2016 John Wiley & Sons Ltd.
Figures





Similar articles
-
[Mage-D1 binding to activated p75NTR positively regulates mineralization of rat ectomesenchymal stem cells in vitro].Nan Fang Yi Ke Da Xue Xue Bao. 2021 Oct 20;41(10):1547-1553. doi: 10.12122/j.issn.1673-4254.2021.10.14. Nan Fang Yi Ke Da Xue Xue Bao. 2021. PMID: 34755671 Free PMC article. Chinese.
-
p75NTR-/- mice exhibit an alveolar bone loss phenotype and inhibited PI3K/Akt/β-catenin pathway.Cell Prolif. 2020 Apr;53(4):e12800. doi: 10.1111/cpr.12800. Epub 2020 Mar 25. Cell Prolif. 2020. PMID: 32215984 Free PMC article.
-
Dynamic expression of Mage-D1 in rat dental germs and potential role in mineralization of ectomesenchymal stem cells.Sci Rep. 2022 Dec 30;12(1):22615. doi: 10.1038/s41598-022-27197-5. Sci Rep. 2022. PMID: 36585447 Free PMC article.
-
The spatiotemporal expression and mineralization regulation of p75 neurotrophin receptor in the early tooth development.Cell Prolif. 2019 Jan;52(1):e12523. doi: 10.1111/cpr.12523. Epub 2018 Oct 25. Cell Prolif. 2019. PMID: 30357966 Free PMC article.
-
Clinical Relevance of a Candidate Stem Cell Marker, p75 Neurotrophin Receptor (p75NTR) Expression in Circulating Tumor Cells.Adv Exp Med Biol. 2017;994:247-254. doi: 10.1007/978-3-319-55947-6_13. Adv Exp Med Biol. 2017. PMID: 28560678 Review.
Cited by
-
P75 neurotrophin receptor positively regulates the odontogenic/osteogenic differentiation of ectomesenchymal stem cells via nuclear factor kappa-B signaling pathway.Bioengineered. 2022 Apr;13(4):11201-11213. doi: 10.1080/21655979.2022.2063495. Bioengineered. 2022. PMID: 35485233 Free PMC article.
-
p75NTR optimizes the osteogenic potential of human periodontal ligament stem cells by up-regulating α1 integrin expression.J Cell Mol Med. 2020 Jul;24(13):7563-7575. doi: 10.1111/jcmm.15390. Epub 2020 May 18. J Cell Mol Med. 2020. PMID: 32424966 Free PMC article.
-
Single-cell analysis of ecto-mesenchymal cells derived from the neural crest reveals the regulatory role of P75NTR in odontogenic and osteogenic differentiation.BMC Oral Health. 2025 Jul 3;25(1):1089. doi: 10.1186/s12903-025-06399-z. BMC Oral Health. 2025. PMID: 40611059 Free PMC article.
-
[Mage-D1 binding to activated p75NTR positively regulates mineralization of rat ectomesenchymal stem cells in vitro].Nan Fang Yi Ke Da Xue Xue Bao. 2021 Oct 20;41(10):1547-1553. doi: 10.12122/j.issn.1673-4254.2021.10.14. Nan Fang Yi Ke Da Xue Xue Bao. 2021. PMID: 34755671 Free PMC article. Chinese.
-
p75NTR-/- mice exhibit an alveolar bone loss phenotype and inhibited PI3K/Akt/β-catenin pathway.Cell Prolif. 2020 Apr;53(4):e12800. doi: 10.1111/cpr.12800. Epub 2020 Mar 25. Cell Prolif. 2020. PMID: 32215984 Free PMC article.
References
-
- Chai Y, Jiang X, Ito Y, et al. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. - PubMed
-
- Mantesso A, Sharpe P. Dental stem cells for tooth regeneration and repair. Expert Opin Biol Ther. 2009;9:1143–1154. - PubMed
-
- Michaeli‐Geller G, Zigdon‐Giladi H. [Bone regeneration induced by stem cells–recent research and future outlook]. Refu'at ha‐peh veha‐shinayim. 2015;32:13–20, 59. - PubMed
-
- Miletich I, Sharpe PT. Neural crest contribution to mammalian tooth formation. Birth Defects Res C Embryo Today. 2004;72:200–212. - PubMed
-
- Kapadia H, Mues G, D'Souza R. Genes affecting tooth morphogenesis. Orthod Craniofac Res. 2007;10:237–244. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

