Engineering a minimal G protein to facilitate crystallisation of G protein-coupled receptors in their active conformation
- PMID: 27672048
- PMCID: PMC5181381
- DOI: 10.1093/protein/gzw049
Engineering a minimal G protein to facilitate crystallisation of G protein-coupled receptors in their active conformation
Abstract
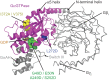
G protein-coupled receptors (GPCRs) modulate cytoplasmic signalling in response to extracellular stimuli, and are important therapeutic targets in a wide range of diseases. Structure determination of GPCRs in all activation states is important to elucidate the precise mechanism of signal transduction and to facilitate optimal drug design. However, due to their inherent instability, crystallisation of GPCRs in complex with cytoplasmic signalling proteins, such as heterotrimeric G proteins and β-arrestins, has proved challenging. Here, we describe the design of a minimal G protein, mini-Gs, which is composed solely of the GTPase domain from the adenylate cyclase stimulating G protein Gs Mini-Gs is a small, soluble protein, which efficiently couples GPCRs in the absence of Gβγ subunits. We engineered mini-Gs, using rational design mutagenesis, to form a stable complex with detergent-solubilised β1-adrenergic receptor (β1AR). Mini G proteins induce similar pharmacological and structural changes in GPCRs as heterotrimeric G proteins, but eliminate many of the problems associated with crystallisation of these complexes, specifically their large size, conformational dynamics and instability in detergent. They are therefore novel tools, which will facilitate the biochemical and structural characterisation of GPCRs in their active conformation.
Keywords: G protein; G protein-coupled receptor; GPCR; Gs; complex; mini G protein; mini-Gs.
© The Author 2016. Published by Oxford University Press.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

