Home-based rehabilitation for COPD using minimal resources: a randomised, controlled equivalence trial
- PMID: 27672116
- PMCID: PMC5329049
- DOI: 10.1136/thoraxjnl-2016-208514
Home-based rehabilitation for COPD using minimal resources: a randomised, controlled equivalence trial
Abstract
Background: Pulmonary rehabilitation is a cornerstone of care for COPD but uptake of traditional centre-based programmes is poor. We assessed whether home-based pulmonary rehabilitation, delivered using minimal resources, had equivalent outcomes to centre-based pulmonary rehabilitation.
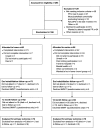
Methods: A randomised controlled equivalence trial with 12 months follow-up. Participants with stable COPD were randomly assigned to receive 8 weeks of pulmonary rehabilitation by either the standard outpatient centre-based model, or a new home-based model including one home visit and seven once-weekly telephone calls from a physiotherapist. The primary outcome was change in 6 min walk distance (6MWD).
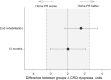
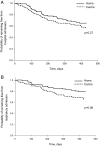
Results: We enrolled 166 participants to receive centre-based rehabilitation (n=86) or home-based rehabilitation (n=80). Intention-to-treat analysis confirmed non-inferiority of home-based rehabilitation for 6MWD at end-rehabilitation and the confidence interval (CI) did not rule out superiority (mean difference favouring home group 18.6 m, 95% CI -3.3 to 40.7). At 12 months the CI did not exclude inferiority (-5.1 m, -29.2 to 18.9). Between-group differences for dyspnoea-related quality of life did not rule out superiority of home-based rehabilitation at programme completion (1.6 points, -0.3 to 3.5) and groups were equivalent at 12 months (0.05 points, -2.0 to 2.1). The per-protocol analysis showed the same pattern of findings. Neither group maintained postrehabilitation gains at 12 months.
Conclusions: This home-based pulmonary rehabilitation model, delivered with minimal resources, produced short-term clinical outcomes that were equivalent to centre-based pulmonary rehabilitation. Neither model was effective in maintaining gains at 12 months. Home-based pulmonary rehabilitation could be considered for people with COPD who cannot access centre-based pulmonary rehabilitation.
Trial registration number: NCT01423227, clinicaltrials.gov.
Keywords: Pulmonary Rehabilitation.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Figures


Comment in
-
Expanding pulmonary rehabilitation capacity. One size won't fit all.Thorax. 2017 Jan;72(1):4-5. doi: 10.1136/thoraxjnl-2016-209345. Epub 2016 Nov 2. Thorax. 2017. PMID: 27807017 No abstract available.
-
Critically Appraised Papers: In people with chronic obstructive pulmonary disease, home-based pulmonary rehabilitation produces similar results to a hospital-based outpatient program [synopsis].J Physiother. 2018 Apr;64(2):122. doi: 10.1016/j.jphys.2018.02.003. Epub 2018 Mar 16. J Physiother. 2018. PMID: 29555422 No abstract available.
-
Critically Appraised Papers: In people with chronic obstructive pulmonary disease, home-based pulmonary rehabilitation produces similar results to a hospital-based outpatient program [commentary].J Physiother. 2018 Apr;64(2):122. doi: 10.1016/j.jphys.2018.02.004. Epub 2018 Mar 19. J Physiother. 2018. PMID: 29567380 No abstract available.
References
-
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2014. http://www.goldcopd.org/ (accessed 26 Jun 2014).
-
- Marks G, Reddel H, Guevara-Rattray E, et al. . Monitoring pulmonary rehabilitation and long-term oxygen therapy for people with COPD in Australia: a discussion paper. Canberra: Australian Institute of Health and Welfare (AIHW), 2013.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
