Nintedanib in patients with idiopathic pulmonary fibrosis and preserved lung volume
- PMID: 27672117
- PMCID: PMC5520269
- DOI: 10.1136/thoraxjnl-2016-208710
Nintedanib in patients with idiopathic pulmonary fibrosis and preserved lung volume
Abstract
Rationale: There is no consensus as to when treatment for idiopathic pulmonary fibrosis (IPF) should be initiated. Some physicians prefer not to treat patients with preserved lung volume.
Objective: To investigate whether patients with IPF and preserved lung volume receive the same benefit from nintedanib as patients with more impaired lung volume.
Methods: Post hoc subgroup analyses of pooled data from the two replicate phase III INPULSIS trials by baseline FVC % predicted (≤90%, >90%).
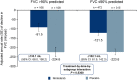
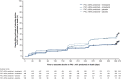
Results: At baseline, 274 patients had FVC >90% predicted and 787 patients had FVC ≤90% predicted. In patients treated with placebo, the adjusted annual rate of decline in FVC was consistent between patients with FVC >90% predicted and FVC ≤90% predicted (-224.6 mL/year and -223.6 mL/year, respectively). There was no statistically significant difference between these subgroups in the effect of nintedanib on annual rate of decline in FVC, change from baseline in St George's Respiratory Questionnaire total score or time to first acute exacerbation. In patients with baseline FVC >90% predicted and ≤90% predicted, respectively, the adjusted annual rate of decline in FVC with nintedanib was -91.5 mL/year (difference vs placebo: 133.1 mL/year (95% CI 68.0 to 198.2)) and -121.5 mL/year (difference vs placebo: 102.1 mL/year (95% CI 61.9 to 142.3)). Adverse events associated with nintedanib were similar in both subgroups.
Conclusions: Patients with IPF and preserved lung volume (FVC >90% predicted) have the same rate of FVC decline and receive the same benefit from nintedanib as patients with more impaired lung volume.
Trial registration number: NCT01335464 and NCT01335477.
Keywords: Idiopathic pulmonary fibrosis.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
