West Nile Virus Infection in Human and Mouse Cornea Tissue
- PMID: 27672204
- PMCID: PMC5094237
- DOI: 10.4269/ajtmh.16-0256
West Nile Virus Infection in Human and Mouse Cornea Tissue
Abstract
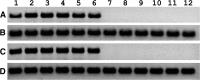
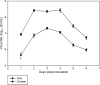
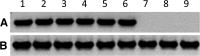
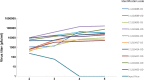
The purpose of this study was to determine the in vitro and ex vivo susceptibility of human corneal cells to West Nile virus (WNV) infection and evaluate the ability of the virus to disseminate to the corneas of infected mice. Human corneal epithelial cells were challenged with WNV, incubated for 1-6 days, and tested for evidence of WNV infection. Viral RNA and antigen were detected at every time point, and the virus reached a peak titer of 2.5 × 107 plaque-forming units (pfu)/mL at 3 days postinoculation (PI). Corneas procured from donors were incubated in culture dishes containing WNV for 1-5 days and tested for evidence of WNV. Viral RNA and antigen were detected, and the virus reached a mean peak titer of 4.9 × 104 pfu/mL at 5 days PI. Mice were inoculated intraperitoneally with WNV, and their eyes were harvested at 2, 5, and 8 days PI and tested for evidence of WNV. Viral RNA was detected in corneas of four of nine systemically infected mice as early as 2 days PI. We conclude that human corneal cells support WNV replication in vitro and ex vivo, and WNV may disseminate into the corneas of experimentally infected mice. These findings indicate that corneal transmission cannot be ruled out as a novel mode of human-to-human WNV transmission and additional experiments should be conducted to assess this risk further.
© The American Society of Tropical Medicine and Hygiene.
Figures

Similar articles
-
Evidence for West Nile virus spillover into the squirrel population in Atlanta, Georgia.Vector Borne Zoonotic Dis. 2015 May;15(5):303-10. doi: 10.1089/vbz.2014.1734. Vector Borne Zoonotic Dis. 2015. PMID: 25988439
-
Identification of West Nile virus infection by anti-premembrane antibodies in Nicaraguan children prior to 2007-2009.Microbiol Spectr. 2025 Jul;13(7):e0004725. doi: 10.1128/spectrum.00047-25. Epub 2025 May 22. Microbiol Spectr. 2025. PMID: 40401969 Free PMC article.
-
Eilat virus (EILV) causes superinfection exclusion against West Nile virus (WNV) in a strain-specific manner in Culex tarsalis mosquitoes.J Gen Virol. 2024 Aug;105(8):002017. doi: 10.1099/jgv.0.002017. J Gen Virol. 2024. PMID: 39189607
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
Patching for corneal abrasion.Cochrane Database Syst Rev. 2016 Jul 26;7(7):CD004764. doi: 10.1002/14651858.CD004764.pub3. Cochrane Database Syst Rev. 2016. PMID: 27457359 Free PMC article.
Cited by
-
Interferon-stimulated gene 15 (ISG15) restricts Zika virus replication in primary human corneal epithelial cells.Ocul Surf. 2019 Jul;17(3):551-559. doi: 10.1016/j.jtos.2019.03.006. Epub 2019 Mar 22. Ocul Surf. 2019. PMID: 30905842 Free PMC article.
References
-
- Zou S, Foster GA, Dodd RY, Petersen LR, Stramer SL. West Nile fever characteristics among viremic persons identified through blood donor screening. J Infect Dis. 2010;202:1354–1361. - PubMed
-
- Lanciotti RS, Roehrig JT, Deubel V, Smith J, Parker M, Steele K, Crise B, Volpe KE, Crabtree MB, Scherret JH, Hall RA, MacKenzie JS, Cropp CB, Panigrahy B, Ostlund E, Schmitt B, Malkinson M, Banet C, Weissman J, Komar N, Savage HM, Stone W, McNamara T, Gubler DJ. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science. 1999;286:2333–2337. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

