Therapeutic aspects of c-MYC signaling in inflammatory and cancerous colonic diseases
- PMID: 27672289
- PMCID: PMC5028808
- DOI: 10.3748/wjg.v22.i35.7938
Therapeutic aspects of c-MYC signaling in inflammatory and cancerous colonic diseases
Abstract
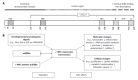
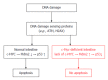
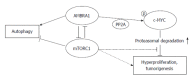
Colonic inflammation is required to heal infections, wounds, and maintain tissue homeostasis. As the seventh hallmark of cancer, however, it may affect all phases of tumor development, including tumor initiation, promotion, invasion and metastatic dissemination, and also evasion immune surveillance. Inflammation acts as a cellular stressor and may trigger DNA damage or genetic instability, and, further, chronic inflammation can provoke genetic mutations and epigenetic mechanisms that promote malignant cell transformation. Both sporadical and colitis-associated colorectal carcinogenesis are multi-step, complex processes arising from the uncontrolled proliferation and spreading of malignantly transformed cell clones with the obvious ability to evade the host's protective immunity. In cells upon DNA damage several proto-oncogenes, including c-MYC are activated in parelell with the inactivation of tumor suppressor genes. The target genes of the c-MYC protein participate in different cellular functions, including cell cycle, survival, protein synthesis, cell adhesion, and micro-RNA expression. The transcriptional program regulated by c-MYC is context dependent, therefore the final cellular response to elevated c-MYC levels may range from increased proliferation to augmented apoptosis. Considering physiological intestinal homeostasis, c-MYC displays a fundamental role in the regulation of cell proliferation and crypt cell number. However, c-MYC gene is frequently deregulated in inflammation, and overexpressed in both sporadic and colitis-associated colon adenocarcinomas. Recent results demonstrated that endogenous c-MYC is essential for efficient induction of p53-dependent apoptosis following DNA damage, but c-MYC function is also involved in and regulated by autophagy-related mechanisms, while its expression is affected by DNA-methylation, or histone acetylation. Molecules directly targeting c-MYC, or agents acting on other genes involved in the c-MYC pathway could be selected for combined regiments. However, due to its context-dependent cellular function, it is clinically essential to consider which cytotoxic drugs are used in combination with c-MYC targeted agents in various tissues. Increasing our knowledge about MYC-dependent pathways might provide direction to novel anti-inflammatory and colorectal cancer therapies.
Keywords: Apoptosis; Autophagy; Colon; Colorectal cancer; Inflammation; Therapy; c-MYC.
Conflict of interest statement
Conflict-of-interest statement: All authors declare no conflict of interest.
Figures
References
-
- Lazebnik Y. What are the hallmarks of cancer? Nat Rev Cancer. 2010;10:232–233. - PubMed
-
- Basith S, Manavalan B, Yoo TH, Kim SG, Choi S. Roles of toll-like receptors in cancer: a double-edged sword for defense and offense. Arch Pharm Res. 2012;35:1297–1316. - PubMed
-
- Mukherjee B, Morgenbesser SD, DePinho RA. Myc family oncoproteins function through a common pathway to transform normal cells in culture: cross-interference by Max and trans-acting dominant mutants. Genes Dev. 1992;6:1480–1492. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

