Second-generation direct-acting-antiviral hepatitis C virus treatment: Efficacy, safety, and predictors of SVR12
- PMID: 27672299
- PMCID: PMC5028818
- DOI: 10.3748/wjg.v22.i35.8050
Second-generation direct-acting-antiviral hepatitis C virus treatment: Efficacy, safety, and predictors of SVR12
Abstract
Aim: To gather data on the antiviral efficacy and safety of second generation direct acting antiviral (DAA) treatment with respect to sustained virological response (SVR) 12 wk after conclusion of treatment, and to determine predictors of SVR12 in this setting.
Methods: Two hundred and sixty patients treated with SOF combination partners PR (n = 51), R (n = 10), SMV (n = 30), DCV (n = 81), LDV (n = 73), or 3D (n = 15). 144/260 were pre-treated, 89/260 had liver cirrhosis, 56/260 had portal hypertension with platelets < 100/nL, 25/260 had a MELD score ≥ 10 and 17/260 were post-liver transplantation patients. 194/260 had HCV GT1, 44/260 HCV GT3.
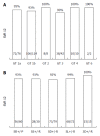
Results: Two hundred and forty/256 (93.7%) patients achieved SVR12 (mITT); 4/260 were lost to follow-up. SVR12 rates for subgroups were: 92% for SOF/DCV, 93% for each SOF/SMV, SOF/PR, 94% for SOF/LDV, 100% for 3D, 94% for pretreated, 87% for liver cirrhosis, 82% for patients with platelets < 100/nL, 88% post-liver transplantation, 95% for GT1a, 93% for GT1b, 90% for GT3, 100% for GT2, 4, and 6. 12 patients suffered from relapse, 6 prematurely discontinued treatment, of which 4 died. Negative predictors of SVR12 were a platelet count < 100/nL, MELD score ≥ 10 (P < 0.0001), liver cirrhosis (P = 0.005) at baseline. In Interferon-free treatment GT3 had significantly lower SVR rates than GT1 (P = 0.016). Side effects were mild.
Conclusion: Excellent SVR12 rates and the favorable side-effect profile of DAA-combination therapy can be well translated into "real-world". Patients with advanced liver disease, signs of portal hypertension, especially with platelets < 100/nL and patients with GT3 are in special need for further research efforts to overcome comparatively higher rates of virological failure.
Keywords: Daclatasvir; Hepatitis C; Ledipasvir; Liver cirrhosis; Liver transplant; Resistance; Side effects; Simeprevir; Sofosbuvir; Sustained virological response.
Conflict of interest statement
Conflict-of-interest statement: Werner CR received travel grants from Gilead, Bristol-Myers-Squibb, Roche, and Janssen-Cilag, and lecture fees from Roche; Schwarz JM and Beck R declare no conflict; Egetemeyr DP received travel grants, and lecture fees from Roche; Malek NP declares no conflict; Lauer UM received travel grants from Roche; Berg CP received travel grants from Gilead, Bristol-Myers-Squibb, Roche, and Janssen-Cilag, and lecture fees from Gilead, Bristol-Myers-Squibb, Roche, and Janssen-Cilag. The authors received no financial support. No funding source exists.
Figures

References
-
- Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29 Suppl 1:74–81. - PubMed
-
- Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. - PubMed
-
- Esteban JI, Sauleda S, Quer J. The changing epidemiology of hepatitis C virus infection in Europe. J Hepatol. 2008;48:148–162. - PubMed
-
- Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45–S57. - PubMed
-
- Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

