Therapeutic efficacy of monthly subcutaneous injection of daclizumab in relapsing multiple sclerosis
- PMID: 27672308
- PMCID: PMC5026217
- DOI: 10.2147/BTT.S89218
Therapeutic efficacy of monthly subcutaneous injection of daclizumab in relapsing multiple sclerosis
Abstract
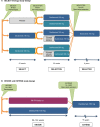
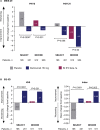
Despite the availability of multiple disease-modifying therapies for relapsing multiple sclerosis (MS), there remains a need for highly efficacious targeted therapy with a favorable benefit-risk profile and attributes that encourage a high level of treatment adherence. Daclizumab is a humanized monoclonal antibody directed against CD25, the α subunit of the high-affinity interleukin 2 (IL-2) receptor, that reversibly modulates IL-2 signaling. Daclizumab treatment leads to antagonism of proinflammatory, activated T lymphocyte function and expansion of immunoregulatory CD56(bright) natural killer cells, and has the potential to, at least in part, rectify the imbalance between immune tolerance and autoimmunity in relapsing MS. The clinical pharmacology, efficacy, and safety of subcutaneous daclizumab have been evaluated extensively in a large clinical study program. In pivotal studies, daclizumab demonstrated superior efficacy in reducing clinical and radiologic measures of MS disease activity compared with placebo or intramuscular interferon beta-1a, a standard-of-care therapy for relapsing MS. The risk of hepatic disorders, cutaneous events, and infections was modestly increased. The monthly subcutaneous self-injection dosing regimen of daclizumab may be advantageous in maintaining patient adherence to treatment, which is important for optimal outcomes with MS disease-modifying therapy. Daclizumab has been approved in the US and in the European Union and represents an effective new treatment option for patients with relapsing forms of MS, and is currently under review by other regulatory agencies.
Keywords: daclizumab; disease-modifying therapy; humanized monoclonal antibody; relapsing multiple sclerosis; therapeutic use.
Figures

Similar articles
-
Daclizumab: A Review in Relapsing Multiple Sclerosis.Drugs. 2017 Mar;77(4):447-458. doi: 10.1007/s40265-017-0708-2. Drugs. 2017. PMID: 28211007 Review.
-
Pharmacokinetic drug evaluation of daclizumab for the treatment of relapsing-remitting multiple sclerosis.Expert Opin Drug Metab Toxicol. 2018 Mar;14(3):341-352. doi: 10.1080/17425255.2018.1432594. Epub 2018 Jan 30. Expert Opin Drug Metab Toxicol. 2018. PMID: 29363337 Review.
-
Daclizumab: Development, Clinical Trials, and Practical Aspects of Use in Multiple Sclerosis.Neurotherapeutics. 2017 Oct;14(4):842-858. doi: 10.1007/s13311-017-0553-8. Neurotherapeutics. 2017. PMID: 28707278 Free PMC article. Review.
-
The efficacy and safety of daclizumab and its potential role in the treatment of multiple sclerosis.Ther Adv Neurol Disord. 2014 Jan;7(1):7-21. doi: 10.1177/1756285613504021. Ther Adv Neurol Disord. 2014. PMID: 24409199 Free PMC article. Review.
-
Daclizumab high-yield process in the treatment of relapsing-remitting multiple sclerosis.Ther Adv Neurol Disord. 2017 Jan;10(1):67-75. doi: 10.1177/1756285616671887. Epub 2016 Oct 19. Ther Adv Neurol Disord. 2017. PMID: 28450896 Free PMC article. Review.
Cited by
-
Progress in the Application of Drugs for the Treatment of Multiple Sclerosis.Front Pharmacol. 2021 Jul 13;12:724718. doi: 10.3389/fphar.2021.724718. eCollection 2021. Front Pharmacol. 2021. PMID: 34326775 Free PMC article. Review.
-
Safety and efficacy of daclizumab beta in patients with relapsing multiple sclerosis in a 5-year open-label study (EXTEND): final results following early termination.Ther Adv Neurol Disord. 2021 Feb 26;14:1756286420987941. doi: 10.1177/1756286420987941. eCollection 2021. Ther Adv Neurol Disord. 2021. PMID: 33737954 Free PMC article.
-
Long-term safety and efficacy of daclizumab beta in relapsing-remitting multiple sclerosis: 6-year results from the SELECTED open-label extension study.J Neurol. 2020 Oct;267(10):2851-2864. doi: 10.1007/s00415-020-09835-y. Epub 2020 May 25. J Neurol. 2020. PMID: 32451615 Free PMC article. Clinical Trial.
References
-
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502–1517. - PubMed
-
- Multiple Sclerosis International Federation Atlas of MS. 2013. [Accessed August 26, 2015]. Available from: http://www.msif.org/about-us/advocacy/atlas/
-
- Freedman MS. Disease-modifying drugs for multiple sclerosis: current and future aspects. Expert Opin Pharmacother. 2006;7(Suppl 1):S1–S9. - PubMed
-
- Lublin FD. The incomplete nature of multiple sclerosis relapse resolution. J Neurol Sci. 2007;256(Suppl 1):S14–S18. - PubMed
-
- Miller DM, Weinstock-Guttman B, Béthoux F, et al. A meta-analysis of methylprednisolone in recovery from multiple sclerosis exacerbations. Mult Scler. 2000;6(4):267–273. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

