Cytocompatibility with osteogenic cells and enhanced in vivo anti-infection potential of quaternized chitosan-loaded titania nanotubes
- PMID: 27672479
- PMCID: PMC5028847
- DOI: 10.1038/boneres.2016.27
Cytocompatibility with osteogenic cells and enhanced in vivo anti-infection potential of quaternized chitosan-loaded titania nanotubes
Abstract
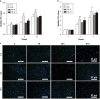
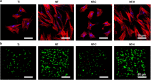
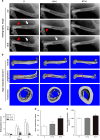
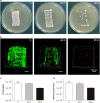
Infection is one of the major causes of failure of orthopedic implants. Our previous study demonstrated that nanotube modification of the implant surface, together with nanotubes loaded with quaternized chitosan (hydroxypropyltrimethyl ammonium chloride chitosan, HACC), could effectively inhibit bacterial adherence and biofilm formation in vitro. Therefore, the aim of this study was to further investigate the in vitro cytocompatibility with osteogenic cells and the in vivo anti-infection activity of titanium implants with HACC-loaded nanotubes (NT-H). The titanium implant (Ti), nanotubes without polymer loading (NT), and nanotubes loaded with chitosan (NT-C) were fabricated and served as controls. Firstly, we evaluated the cytocompatibility of these specimens with human bone marrow-derived mesenchymal stem cells in vitro. The observation of cell attachment, proliferation, spreading, and viability in vitro showed that NT-H has improved osteogenic activity compared with Ti and NT-C. A prophylaxis rat model with implantation in the femoral medullary cavity and inoculation with methicillin-resistant Staphylococcus aureus was established and evaluated by radiographical, microbiological, and histopathological assessments. Our in vivo study demonstrated that NT-H coatings exhibited significant anti-infection capability compared with the Ti and NT-C groups. In conclusion, HACC-loaded nanotubes fabricated on a titanium substrate show good compatibility with osteogenic cells and enhanced anti-infection ability in vivo, providing a good foundation for clinical application to combat orthopedic implant-associated infections.
Figures




References
-
- Court-Brown CM, Keating JF, McQueen MM. Infection after intramedullary nailing of the tibia. Incidence and protocol for management. J Bone Joint Surg Br 1992; 74: 770–774. - PubMed
-
- Chen CE, Ko JY, Wang JW et al. Infection after intramedullary nailing of the femur. J Trauma 2003; 55: 338–344. - PubMed
-
- Court-Brown CM. Reamed intramedullary tibial nailing: an overview and analysis of 1106 Cases. J Orthop Trauma 2004; 18: 96–101. - PubMed
-
- Birdsall PD, Milne DD. Toxic shock syndrome due to percutaneous Kirschner wires. Injury 1999; 30: 509–510. - PubMed
-
- Losic D, Aw MS, Santos A et al. Titania nanotube arrays for local drug delivery: recent advances and perspectives. Expert Opin Drug Deliv 2015; 12: 103–127. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

