Pharmaceutical Innovation in the Treatment of Schizophrenia and Mental Disorders Compared with Other Diseases
- PMID: 27672484
- PMCID: PMC5022985
Pharmaceutical Innovation in the Treatment of Schizophrenia and Mental Disorders Compared with Other Diseases
Abstract
Objectives: The objectives of this study were to assess the level of private and public investment in research and development of treatments for schizophrenia and other mental disorders compared to other diseases in order to present data on the economic burden and pharmaceutical innovation by disease area, and to compare the level of investment relative to burden across different diseases.
Design: The levels of investment and pharmaceutical innovation relative to burden across different diseases were assessed. Disease burden and prevalence for mental disorders (schizophrenia, bipolar disorder, and major depressive disorder); cancer; rheumatoid arthritis; chronic obstructive pulmonary disorder; diabetes; cardiovascular disease; and neurological disorders (dementia and epilepsy) were estimated from literature sources.
Setting: Pharmaceutical treatment innovation was measured by the total number of drug launches and the number of drugs launched categorized by innovativeness. Research and development expenditures were estimated using published information on annual public and domestic private research and development expenditures by disease area. Lastly, investment relative to disease burden was measured among the set of disease classes for which all three measures were available: schizophrenia, bipolar disorder, major depressive disorder, cancer, rheumatoid arthritis, chronic obstructive pulmonary disease, diabetes, cardiovascular disease, and neurology (dementia and epilepsy combined).
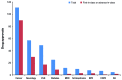
Results: The level of investment and pharmaceutical innovation in mental disorders was comparatively low, especially relative to the burden of disease. For mental disorders, investment was $3.1 per $1,000 burden invested in research and development for schizophrenia, $1.8 for major depressive disorder, and $0.4 for bipolar disorder relative to cancer ($75.5), chronic obstructive pulmonary disease ($9.4), diabetes ($7.6), cardiovascular disease ($6.3), or rheumatoid arthritis ($5.3). Pharmaceutical innovation was also low for mental disorders.
Conclusion: Despite the significant burden mental disorders impose on society, investment and pharmaceutical innovation in this disease area remains comparatively low. Policymakers should consider new strategies to stimulate public and private investment in the research and development of novel and effective therapies to treat schizophrenia and other mental disorders.
Keywords: Mental disorders; bipolar disorder; disease burden; expenditures; major depressive disorder; pharmaceutical innovation; research and development; schizophrenia.
Figures

References
-
- Philipson T, Jena A. Who benefits from new medical technologies? Estimates of consumer and producer surpluses for HIV/AIDS drugs. National Bureau of Economic Research. Working Paper No. 11810. December, 2005.
-
- Grabowski D, Lakdawalla D, Goldman D, et al. The large social value resulting from use of statins warrants steps to improve adherence and broaden treatment. Health Aff. 2012;31(10):2276–2285. - PubMed
-
- Yin P, MacLean R, Lakdawalla D, Philipson T. Value of survival gains in chronic myeloid leukemia. Am J Manag Care. 2012;18(suppl 11):257–264. - PubMed
-
- DiMasi J. CNS drugs take longer to develop, have lower success rates, than other drugs. Tufts Center for the Study of Drug Development website. November 4, 2014. [June 5, 2015]. http://csdd.tufts.edu/news/complete_story/pr_ir_nov_dec_ir
LinkOut - more resources
Full Text Sources
