Pre-exposure Prophylaxis Use by Breastfeeding HIV-Uninfected Women: A Prospective Short-Term Study of Antiretroviral Excretion in Breast Milk and Infant Absorption
- PMID: 27676257
- PMCID: PMC5038971
- DOI: 10.1371/journal.pmed.1002132
Pre-exposure Prophylaxis Use by Breastfeeding HIV-Uninfected Women: A Prospective Short-Term Study of Antiretroviral Excretion in Breast Milk and Infant Absorption
Abstract
Background: As pre-exposure prophylaxis (PrEP) becomes more widely used in heterosexual populations, an important consideration is its safety in infants who are breastfed by women taking PrEP. We investigated whether tenofovir and emtricitabine are excreted into breast milk and then absorbed by the breastfeeding infant in clinically significant concentrations when used as PrEP by lactating women.
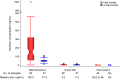
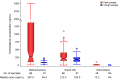
Methods and findings: We conducted a prospective short-term, open-label study of daily oral emtricitabine-tenofovir disoproxil fumarate PrEP among 50 HIV-uninfected breastfeeding African mother-infant pairs between 1-24 wk postpartum (ClinicalTrials.gov Identifier: NCT02776748). The primary goal was to quantify the steady-state concentrations of tenofovir and emtricitabine in infant plasma ingested via breastfeeding. PrEP was administered to women through daily directly observed therapy (DOT) for ten consecutive days and then discontinued thereafter. Non-fasting peak and trough samples of maternal plasma and breast milk were obtained at drug concentration steady states on days 7 and 10, and a single infant plasma sample was obtained on day 7. Peak blood and breast milk samples were obtained 1-2 h after the maternal DOT PrEP dose, while maternal trough samples were obtained at the end of the PrEP dosing interval (i.e., 23 to 24 h) after maternal DOT PrEP dose. Tenofovir and emtricitabine concentrations were quantified using liquid chromatography-tandem mass spectrometry (LC-MS/MS) assays. Of the 50 mother-infant pairs enrolled, 48% were ≤12 wk and 52% were 13-24 wk postpartum, and median maternal age was 25 y (interquartile range [IQR] 22-28). During study follow-up, the median (IQR) daily reported frequency of infant breastfeeding was 15 times (12 to 18) overall, 16 (14 to 19) for the ≤12 weeks, and 14 (12 to 17) for the 13-24 wk infant age groups. Overall, median (IQR) time-averaged peak concentrations in breast milk were 3.2 ng/mL (2.3 to 4.7) for tenofovir and 212.5 ng/mL (140.0 to 405.0) for emtricitabine. Similarly, median (IQR) time-averaged trough concentrations in breast milk were 3.3 ng/mL (2.3 to 4.4) for tenofovir and 183.0 ng/mL (113.0 to 250.0) for emtricitabine, reflecting trough-to-peak breast milk concentration ratios of 1.0 for tenofovir and 0.8 for emtricitabine, respectively. In infant plasma, tenofovir was unquantifiable in 46/49 samples (94%), but emtricitabine was detectable in 47/49 (96%) (median [IQR] concentration: 13.2 ng/mL [9.3 to 16.7]). The estimated equivalent doses an infant would ingest daily from breastfeeding were 0.47 μg/kg (IQR 0.35 to 0.71) for tenofovir and 31.9 μg/kg (IQR 21.0 to 60.8) for emtricitabine, translating into a <0.01% and 0.5% relative dose when compared to the 6 mg/kg dose that is proposed for therapeutic treatment of infant HIV infection and for prevention of infant postnatal HIV infection; a dose that has not shown safety concerns. No serious adverse effects were recorded during study follow-up. The key study limitation was that only a single infant sample was collected to minimize venipunctures for the children. However, maternal daily DOT and specimen collection at drug concentration steady state provided an adequate approach to address the key research question. Importantly, there was minimal variation in breast milk concentrations of tenofovir and emtricitabine (respective median trough-to-peak concentration ratio ~1), demonstrating that infants were exposed to consistent drug dosing via breast milk.
Conclusion: In this short-term study of daily directly observed oral PrEP in HIV-uninfected breastfeeding women, the estimated infant doses from breast milk and resultant infant plasma concentrations for tenofovir and emtricitabine were 12,500 and >200-fold lower than the respective proposed infant therapeutic doses, and tenofovir was not detected in 94% of infant plasma samples. These data suggest that PrEP can be safely used during breastfeeding with minimal infant drug exposure.
Trial registration: ClinicalTrials.gov, Identifier: NCT02776748.
Conflict of interest statement
I have read the journal's policy, and the authors of this manuscript have the following competing interests: FTC-TDF was donated by Gilead Sciences. CWH reports a prior contract from Gilead Sciences outside the submitted work and a patent pending, both managed by Johns Hopkins. MM has grant funding via the NIH. GJS has research grants from NIH (unrelated), CDC (unrelated), Thrasher Foundation (unrelated), Bill and Melinda Gates Foundation (sponsor), royalties from UpToDate (unrelated), and salary support from the University of Washington. All authors declare no other conflicts of interest.
Figures
Similar articles
-
Plasma and breast milk pharmacokinetics of emtricitabine, tenofovir and lamivudine using dried blood and breast milk spots in nursing African mother-infant pairs.J Antimicrob Chemother. 2018 Apr 1;73(4):1013-1019. doi: 10.1093/jac/dkx507. J Antimicrob Chemother. 2018. PMID: 29309634 Free PMC article.
-
Safety and pharmacokinetics of single, dual, and triple antiretroviral drug formulations delivered by pod-intravaginal rings designed for HIV-1 prevention: A Phase I trial.PLoS Med. 2018 Sep 28;15(9):e1002655. doi: 10.1371/journal.pmed.1002655. eCollection 2018 Sep. PLoS Med. 2018. PMID: 30265679 Free PMC article. Clinical Trial.
-
Safety of oral tenofovir disoproxil fumarate-based HIV pre-exposure prophylaxis use in lactating HIV-uninfected women.Expert Opin Drug Saf. 2017 Jul;16(7):867-871. doi: 10.1080/14740338.2017.1338271. Epub 2017 Jun 8. Expert Opin Drug Saf. 2017. PMID: 28571500 Free PMC article. Review.
-
Daily and non-daily pre-exposure prophylaxis in African women (HPTN 067/ADAPT Cape Town Trial): a randomised, open-label, phase 2 trial.Lancet HIV. 2018 Feb;5(2):e68-e78. doi: 10.1016/S2352-3018(17)30156-X. Epub 2017 Oct 3. Lancet HIV. 2018. PMID: 28986029 Free PMC article. Clinical Trial.
-
Tenofovir disoproxil fumarate safety for women and their infants during pregnancy and breastfeeding.AIDS. 2017 Jan 14;31(2):213-232. doi: 10.1097/QAD.0000000000001313. AIDS. 2017. PMID: 27831952 Review.
Cited by
-
Scale up of PrEP integrated in public health HIV care clinics: a protocol for a stepped-wedge cluster-randomized rollout in Kenya.Implement Sci. 2018 Sep 4;13(1):118. doi: 10.1186/s13012-018-0809-7. Implement Sci. 2018. PMID: 30180860 Free PMC article. Clinical Trial.
-
Guidelines to support HIV-affected individuals and couples to achieve pregnancy safely: Update 2018.South Afr J HIV Med. 2018 Oct 18;19(1):915. doi: 10.4102/sajhivmed.v19i1.915. eCollection 2018. South Afr J HIV Med. 2018. PMID: 30473876 Free PMC article. No abstract available.
-
Plasma and Breast Milk Pharmacokinetics of Tenofovir Disoproxil Fumarate in Nursing Mother with Chronic Hepatitis B-Infant Pairs.Antimicrob Agents Chemother. 2021 Sep 17;65(10):e0111021. doi: 10.1128/AAC.01110-21. Epub 2021 Jul 26. Antimicrob Agents Chemother. 2021. PMID: 34310204 Free PMC article.
-
Establishing adherence-concentration-efficacy thresholds of TDF-FTC pre-exposure prophylaxis for HIV prevention in African women: a protocol for the Women TDF-FTC Benchmark Study.Front Reprod Health. 2024 May 27;6:1325257. doi: 10.3389/frph.2024.1325257. eCollection 2024. Front Reprod Health. 2024. PMID: 38860025 Free PMC article.
-
Safety and drug quantification of the dapivirine vaginal ring and oral pre-exposure prophylaxis in breastfeeding mother-infant pairs (MTN-043): a phase 3B, open-label, randomised trial.Lancet HIV. 2025 Mar;12(3):e180-e190. doi: 10.1016/S2352-3018(24)00306-0. Epub 2025 Feb 12. Lancet HIV. 2025. PMID: 39954697 Clinical Trial.
References
-
- UNAIDS. The Gap Report 2014. http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Gap_report_.... Accessed December 21, 2015.
-
- Gray RH, Li X, Kigozi G, Serwadda D, Brahmbhatt H, Wabwire-Mangen F, et al. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet. 2005;366(9492):1182–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

