A Positive Selection for Nucleoside Kinases in E. coli
- PMID: 27677184
- PMCID: PMC5038940
- DOI: 10.1371/journal.pone.0162921
A Positive Selection for Nucleoside Kinases in E. coli
Abstract
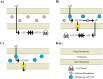
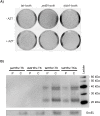
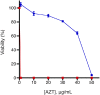
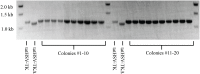
Engineering heterologous nucleoside kinases inside E. coli is a difficult process due to the integral role nucleosides play in cell division and transcription. Nucleoside analogs are used in many kinase screens that depend on cellular metabolization of the analogs. However, metabolic activation of these analogs can be toxic through disruptions of DNA replication and transcription because of the analogs' structural similarities to native nucleosides. Furthermore, the activity of engineered kinases can be masked by endogenous kinases in the cytoplasm, which leads to more difficulties in assessing target activity. A positive selection method that can discern a heterologous kinases' enzymatic activity without significantly influencing the cell's normal metabolic systems would be beneficial. We have developed a means to select for a nucleoside kinase's activity by transporting the kinase to the periplasmic space of an E. coli strain that has its PhoA alkaline phosphatase knocked out. Our proof-of-principle studies demonstrate that the herpes simplex virus thymidine kinase (HSV-TK) can be transported to the periplasmic space in functional form by attaching a tat-signal sequence to the N-terminus of the protein. HSV-TK phosphorylates the toxic nucleoside analog 3'-azido-3'-deoxythymidine (AZT), and this charged, monophosphate form of AZT cannot cross the inner membrane. The translocation of HSV-TK provides significant resistance to AZT when compared to bacteria lacking a periplasmic HSV-TK. However, resistance decreased dramatically above 40 μg/ml AZT. We propose that this threshold can be used to select for higher activity variants of HSV-TK and other nucleoside kinases in a manner that overcomes the efficiency and localization issues of previous selection schemes. Furthermore, our selection strategy should be a general strategy to select or evaluate nucleoside kinases that phosphorylate nucleosides such as prodrugs that would otherwise be toxic to E. coli.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Littler E, Oberg B. Achievements and challenges in antiviral drug discovery. Antivir Chem Chemother. 2005;16: 155–168. - PubMed
-
- Niculescu-Duvaz I, Friedlos F, Niculescu-Duvaz D, Davies L, Springer CJ. Prodrugs for antibody- and gene-directed enzyme prodrug therapies (ADEPT and GDEPT). Anticancer Drug Des. 1999;14: 517–538. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

