Improving GHB withdrawal with baclofen: study protocol for a feasibility study for a randomised controlled trial
- PMID: 27677382
- PMCID: PMC5039898
- DOI: 10.1186/s13063-016-1593-9
Improving GHB withdrawal with baclofen: study protocol for a feasibility study for a randomised controlled trial
Abstract
Background: GHB (gamma-hydroxybutyrate) and its pro-drugs GBL (gamma-butyrolactone) and 1,4-butanediol (1,4-BD) are central nervous system depressants whose street names include 'G' and 'liquid ecstasy'. They are used recreationally predominately for their stimulant and pro-sexual effects or for sedation to help with sleep and/or to 'come down' after using stimulant recreational drugs. Although overall population prevalence is low (0.1 %), in some groups such as men who have sex with men, GHB/GBL use may reach 20 %. GHB/GBL dependence may be associated with severe withdrawal with individuals presenting either acutely to emergency departments or to addiction services for support. Benzodiazepines are currently prescribed for GHB/GBL detoxification but do not prevent all complications, such as behavioural disinhibition, that may require hospitalisation or admission to a high dependency/intensive care unit. The GABAB receptor mediates most effects of GHB/GBL and the GABAB agonist, baclofen, has shown promise as an adjunct to benzodiazepines in reducing withdrawal severity when prescribed both during withdrawal and as a 2-day 'preload' prior to detoxification. The key aim of this feasibility study is provide information about recruitment and characteristics of the proposed outcome measure (symptom severity, complications including delirium and treatment escalation) to inform an application for a definitive randomised placebo controlled trial to determine the role of baclofen in the management of GHB/GBL withdrawal and whether starting baclofen 2 days earlier improves outcomes further.
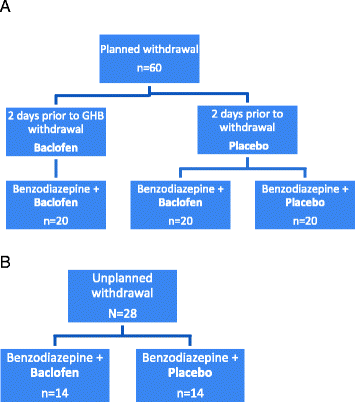
Methods/design: This is a prospective, randomised, double-blind, placebo-controlled feasibility study that will recruit participants (aged over 18 years) who are GHB/GBL-dependent and wish to undergo planned GHB/GBL detoxification or are at risk of acute withdrawal and are inpatients requiring unplanned withdrawal. We aim to recruit 88 participants: 28 unplanned inpatients and 60 planned outpatients. During detoxification we will compare baclofen 10 mg three times a day with placebo as an adjunct to the usual benzodiazepine regimen. In the planned outpatient arm, we will also compare a 2-day preload of baclofen 10 mg three times a day with placebo. Ratings of GHB/GBL withdrawal, sleep, depression, anxiety as well as GHB/GBL use will be collected. The main data analyses will be descriptive about recruitment and characterising the impact of adding baclofen to the usual benzodiazepine regimen on measures and outcomes of GHB/GBL withdrawal to provide estimates of variability and effect size. A qualitative approach will evaluate research participant and clinician acceptability and data collected to inform cost-effectiveness.
Discussion: This feasibility study will inform a randomised controlled trial to establish whether adding baclofen to a benzodiazepine regimen reduces the severity and complications of GHB/GBL withdrawal.
Trial registration: ISRCTN59911189 . Registered 14 October 2015.
Protocol: v3.1, 1 February 2016.
Keywords: Baclofen; Benzodiazepine; GABAB; GBL; GHB; GHB/GBL dependence; GHB/GBL withdrawal; Gamma-butyrolactone; Gamma-hydroxybutyrate.
Figures
Similar articles
-
The clinical toxicology of γ-hydroxybutyrate, γ-butyrolactone and 1,4-butanediol.Clin Toxicol (Phila). 2012 Jul;50(6):458-70. doi: 10.3109/15563650.2012.702218. Clin Toxicol (Phila). 2012. PMID: 22746383 Review.
-
Inpatient management of GHB/GBL withdrawal.Psychiatr Danub. 2019 Sep;31(Suppl 3):354-356. Psychiatr Danub. 2019. PMID: 31488752
-
Inpatient GHB withdrawal management in an inner-city hospital in Sydney, Australia: a retrospective medical record review.Psychopharmacology (Berl). 2023 Jan;240(1):127-135. doi: 10.1007/s00213-022-06283-6. Epub 2022 Dec 12. Psychopharmacology (Berl). 2023. PMID: 36508055 Free PMC article.
-
Cataleptic effects of gamma-hydroxybutyrate (GHB), its precursor gamma-butyrolactone (GBL), and GABAB receptor agonists in mice: differential antagonism by the GABAB receptor antagonist CGP35348.Psychopharmacology (Berl). 2007 Jun;192(3):407-14. doi: 10.1007/s00213-007-0718-y. Epub 2007 Feb 3. Psychopharmacology (Berl). 2007. PMID: 17277933
-
Current Insights on the Impact of Gamma-Hydroxybutyrate (GHB) Abuse.Subst Abuse Rehabil. 2022 Feb 9;13:13-23. doi: 10.2147/SAR.S315720. eCollection 2022. Subst Abuse Rehabil. 2022. PMID: 35173515 Free PMC article. Review.
Cited by
-
Baclofen to Prevent Relapse in Gamma-Hydroxybutyrate (GHB)-Dependent Patients: A Multicentre, Open-Label, Non-Randomized, Controlled Trial.CNS Drugs. 2018 May;32(5):437-442. doi: 10.1007/s40263-018-0516-6. CNS Drugs. 2018. PMID: 29651711 Free PMC article. Clinical Trial.
-
Tapering with Pharmaceutical GHB or Benzodiazepines for Detoxification in GHB-Dependent Patients: A Matched-Subject Observational Study of Treatment-as-Usual in Belgium and The Netherlands.CNS Drugs. 2020 Jun;34(6):651-659. doi: 10.1007/s40263-020-00730-8. CNS Drugs. 2020. PMID: 32319006 Free PMC article.
-
Baclofen in gamma-hydroxybutyrate withdrawal: patterns of use and online availability.Eur J Clin Pharmacol. 2018 Mar;74(3):349-356. doi: 10.1007/s00228-017-2387-z. Epub 2017 Dec 3. Eur J Clin Pharmacol. 2018. PMID: 29198063 Free PMC article. Review.
-
Successful Management of Gamma-hydroxybutyrate (GHB) Withdrawal Using Baclofen as a Standalone Therapy: A Case Report.J Addict Med. 2019 Sep/Oct;13(5):415-417. doi: 10.1097/ADM.0000000000000514. J Addict Med. 2019. PMID: 30907765 Free PMC article.
-
A Comparison Study of Impulsiveness, Cognitive Function, and P300 Components Between Gamma-Hydroxybutyrate and Heroin-Addicted Patients: Preliminary Findings.Front Hum Neurosci. 2022 Apr 20;16:835922. doi: 10.3389/fnhum.2022.835922. eCollection 2022. Front Hum Neurosci. 2022. PMID: 35529779 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources