Genistein suppresses leptin-induced proliferation and migration of vascular smooth muscle cells and neointima formation
- PMID: 27677429
- PMCID: PMC5323876
- DOI: 10.1111/jcmm.12986
Genistein suppresses leptin-induced proliferation and migration of vascular smooth muscle cells and neointima formation
Abstract
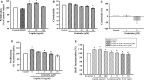
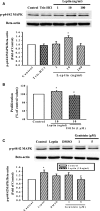
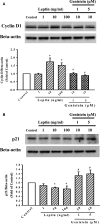
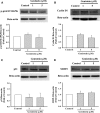
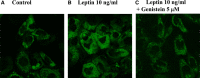
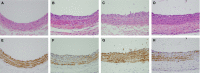
Obesity is a strong risk factor for the development of cardiovascular diseases and is associated with a marked increase in circulating leptin concentration. Leptin is a peptide hormone mainly produced by adipose tissue and is regulated by energy level, hormones and various inflammatory mediators. Genistein is an isoflavone that exhibits diverse health-promoting effects. Here, we investigated whether genistein suppressed the atherogenic effect induced by leptin. The A10 cells were treated with leptin and/or genistein, and then the cell proliferation and migration were analysed. The reactive oxygen species (ROS) and proteins levels were also measured, such as p44/42MAPK, cell cycle-related protein (cyclin D1 and p21) and matrix metalloproteinase-2 (MMP-2). Immunohistochemistry and morphometric analysis were used for the neointima formation in a rat carotid artery injury model. Genistein (5 μM) significantly inhibited both the proliferation and migration of leptin (10 ng/ml)-stimulated A10 cells. In accordance with these finding, genistein decreased the leptin-stimulated ROS production and phosphorylation of the p44/42MAPK signal transduction pathway. Meanwhile, genistein reversed the leptin-induced expression of cyclin D1, and cyclin-dependent kinase inhibitor, p21. Genistein attenuated leptin-induced A10 cell migration by inhibiting MMP-2 activity. Furthermore, the leptin (0.25 mg/kg)-augmented neointima formation in a rat carotid artery injury model was attenuated in the genistein (5 mg/kg body weight)-treated group when compared with the balloon injury plus leptin group. Genistein was capable of suppressing the atherogenic effects of leptin in vitro and in vivo, and may be a promising candidate drug in the clinical setting.
Keywords: Leptin; MMP2; carotid artery injury; genistein; reactive oxygen species.
© 2016 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures

References
-
- Chistiakov DA, Orekhov AN, Bobryshev YV. Vascular smooth muscle cell in atherosclerosis. Acta Physiol (Oxf). 2015; 214: 33–50. - PubMed
-
- Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005; 96: 939–49. - PubMed
-
- Sharma AM. Obesity and cardiovascular risk. Growth Horm IGF Res. 2003; 13 (Suppl A): S10–7. - PubMed
-
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998; 395: 763–70. - PubMed
-
- Fortuño A, Rodríguez A, Gómez‐Ambrosi J, et al Adipose tissue as an endocrine organ: role of leptin and adiponectin in the pathogenesis of cardiovascular diseases. J Physiol Biochem. 2003; 59: 51–60. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

