The Role of Panobinostat Plus Bortezomib and Dexamethasone in Treating Relapsed or Relapsed and Refractory Multiple Myeloma: A European Perspective
- PMID: 27677481
- PMCID: PMC5083773
- DOI: 10.1007/s12325-016-0413-7
The Role of Panobinostat Plus Bortezomib and Dexamethasone in Treating Relapsed or Relapsed and Refractory Multiple Myeloma: A European Perspective
Abstract
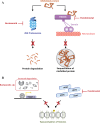
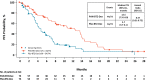
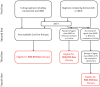
Panobinostat is an oral pan-histone deacetylase inhibitor developed by Novartis. Panobinostat acts via epigenetic modification and inhibition of the aggresome pathway. In August 2015, the European Commission authorized panobinostat for use in combination with bortezomib and dexamethasone for the treatment of relapsed or relapsed and refractory multiple myeloma (MM) in patients who have received ≥2 prior regimens including bortezomib and an immunomodulatory drug. In January 2016, the National Institute for Health and Care Excellence recommended panobinostat for use in the same combination and patient population. The authorization and recommendation were based on results from the pivotal phase 3 PANORAMA 1 (NCT01023308) clinical trial, which demonstrated an improvement in median progression-free survival of 7.8 months for the three-drug combination compared with placebo plus bortezomib and dexamethasone in this patient population. This review will discuss the current treatment landscape for relapsed/refractory MM, the mechanism of action of panobinostat, clinical data supporting the European authorization, concerns about safety and strategies for mitigating toxicity, and how panobinostat fits into the current MM landscape in Europe.
Funding: Editorial support, funded by Novartis Pharmaceuticals.
Keywords: Multiple myeloma; Oncology; Panobinostat; Relapsed; Relapsed and refractory.
Figures
References
-
- International Agency for Research on Cancer. Estimated cancer incidence, mortality, prevalence and disability-adjusted life years (DALYs) worldwide in 2012. GLOBOCAN. 2012. Accessed 25 Jan 2016.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

