Necroptosis is a key mediator of enterocytes loss in intestinal ischaemia/reperfusion injury
- PMID: 27677535
- PMCID: PMC5323854
- DOI: 10.1111/jcmm.12987
Necroptosis is a key mediator of enterocytes loss in intestinal ischaemia/reperfusion injury
Abstract
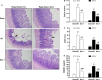
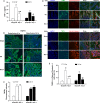
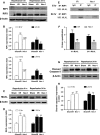
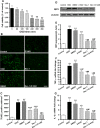
Cell death is an important biological process that is believed to have a central role in intestinal ischaemia/reperfusion (I/R) injury. While the apoptosis inhibition is pivotal in preventing intestinal I/R, how necrotic cell death is regulated remains unknown. Necroptosis represents a newly discovered form of programmed cell death that combines the features of both apoptosis and necrosis, and it has been implicated in the development of a range of inflammatory diseases. Here, we show that receptor-interacting protein 1/3 (RIP1/3) kinase and mixed lineage kinase domain-like protein recruitment mediates necroptosis in a rat model of ischaemic intestinal injury in vivo. Furthermore, necroptosis was specifically blocked by the RIP1 kinase inhibitor necrostatin-1. In addition, the combined treatment of necrostatin-1 and the pan-caspase inhibitor Z-VAD acted synergistically to protect against intestinal I/R injury, and these two pathways can be converted to one another when one is inhibited. In vitro, necrostatin-1 pre-treatment reduced the necroptotic death of oxygen-glucose deprivation challenged intestinal epithelial cell-6 cells, which in turn dampened the production of pro-inflammatory cytokines (tumour necrosis factor-α and interleukin-1β), and suppressed high-mobility group box-1 (HMGB1) translocation from the nucleus to the cytoplasm and the subsequent release of HMGB1 into the supernatant, thus decreasing the activation of Toll-like receptor 4 and the receptor for advanced glycation end products. Collectively, our study reveals a robust RIP1/RIP3-dependent necroptosis pathway in intestinal I/R-induced intestinal injury in vivo and in vitro and suggests that the HMGB1 signalling is highly involved in this process, making it a novel therapeutic target for acute ischaemic intestinal injury.
Keywords: high-mobility group box-1; intestine; ischaemia/reperfusion injury; programmed necrosis.
© 2016 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures


Similar articles
-
Necrostatin-1 Synergizes the Pan Caspase Inhibitor to Attenuate Lung Injury Induced by Ischemia Reperfusion in Rats.Mediators Inflamm. 2020 Oct 24;2020:7059304. doi: 10.1155/2020/7059304. eCollection 2020. Mediators Inflamm. 2020. PMID: 33162831 Free PMC article.
-
Distinct cytoprotective roles of pyruvate and ATP by glucose metabolism on epithelial necroptosis and crypt proliferation in ischaemic gut.J Physiol. 2017 Jan 15;595(2):505-521. doi: 10.1113/JP272208. Epub 2016 Jun 17. J Physiol. 2017. PMID: 27121603 Free PMC article.
-
Inhibition of receptor-interacting protein 3 upregulation and nuclear translocation involved in Necrostatin-1 protection against hippocampal neuronal programmed necrosis induced by ischemia/reperfusion injury.Brain Res. 2015 Jun 3;1609:63-71. doi: 10.1016/j.brainres.2015.03.024. Epub 2015 Mar 20. Brain Res. 2015. PMID: 25801119
-
Necroptosis in neurodegenerative diseases: a potential therapeutic target.Cell Death Dis. 2017 Jun 29;8(6):e2905. doi: 10.1038/cddis.2017.286. Cell Death Dis. 2017. PMID: 28661482 Free PMC article. Review.
-
The possible roles of necroptosis during cerebral ischemia and ischemia / reperfusion injury.Arch Biochem Biophys. 2020 Nov 30;695:108629. doi: 10.1016/j.abb.2020.108629. Epub 2020 Oct 14. Arch Biochem Biophys. 2020. PMID: 33068524 Review.
Cited by
-
Extracellular vesicles derived from bone marrow mesenchymal stem cells regulate SREBF2/HMGB1 axis by transporting miR-378a-3p to inhibit ferroptosis in intestinal ischemia-reperfusion injury.Cell Death Discov. 2025 May 7;11(1):223. doi: 10.1038/s41420-025-02509-6. Cell Death Discov. 2025. PMID: 40335466 Free PMC article.
-
E3 ligase TRIM65 alleviates intestinal ischemia/reperfusion injury through inhibition of TOX4-mediated apoptosis.Cell Death Dis. 2024 Jan 11;15(1):29. doi: 10.1038/s41419-023-06410-x. Cell Death Dis. 2024. PMID: 38212319 Free PMC article.
-
Caspase-1 inhibition prevents glial inflammasome activation and pyroptosis in models of multiple sclerosis.Proc Natl Acad Sci U S A. 2018 Jun 26;115(26):E6065-E6074. doi: 10.1073/pnas.1722041115. Epub 2018 Jun 12. Proc Natl Acad Sci U S A. 2018. PMID: 29895691 Free PMC article.
-
The Contribution of Necroptosis in Neurodegenerative Diseases.Neurochem Res. 2017 Aug;42(8):2117-2126. doi: 10.1007/s11064-017-2249-1. Epub 2017 Apr 5. Neurochem Res. 2017. PMID: 28382594 Review.
-
Necrostatin-1 Synergizes the Pan Caspase Inhibitor to Attenuate Lung Injury Induced by Ischemia Reperfusion in Rats.Mediators Inflamm. 2020 Oct 24;2020:7059304. doi: 10.1155/2020/7059304. eCollection 2020. Mediators Inflamm. 2020. PMID: 33162831 Free PMC article.
References
-
- Mallick IH, Yang W, Winslet MC, et al Ischemia‐reperfusion injury of the intestine and protective strategies against injury. Dig Dis Sci. 2004; 49: 1359–77. - PubMed
-
- Martin B. Prevention of gastrointestinal complications in the critically ill patient. AACN Adv Crit Care. 2007; 18: 158–66. - PubMed
-
- Acosta S. Epidemiology of mesenteric vascular disease: clinical implications. Semin Vasc Surg. 2010; 23: 4–8. - PubMed
-
- Yasuhara H. Acute mesenteric ischemia: the challenge of gastroenterology. Surg Today. 2005; 35: 185–95. - PubMed
-
- Liu KX, Chen SQ, Huang WQ, et al Propofol pretreatment reduces ceramide production and attenuates intestinal mucosal apoptosis induced by intestinal ischemia/reperfusion in rats. Anesth Analg. 2008; 107: 1884–91. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

