Liver injury from herbal and dietary supplements
- PMID: 27677775
- PMCID: PMC5502701
- DOI: 10.1002/hep.28813
Liver injury from herbal and dietary supplements
Abstract
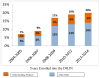
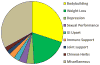
Herbal and dietary supplements (HDS) are used increasingly both in the United States and worldwide, and HDS-induced liver injury in the United States has increased proportionally. Current challenges in the diagnosis and management of HDS-induced liver injury were the focus of a 2-day research symposium sponsored by the American Association for the Study of Liver Disease and the National Institutes of Health. HDS-induced liver injury now accounts for 20% of cases of hepatotoxicity in the United States based on research data. The major implicated agents include anabolic steroids, green tea extract, and multi-ingredient nutritional supplements. Anabolic steroids marketed as bodybuilding supplements typically induce a prolonged cholestatic but ultimately self-limiting liver injury that has a distinctive serum biochemical as well as histological phenotype. Green tea extract and many other products, in contrast, tend to cause an acute hepatitis-like injury. Currently, however, the majority of cases of HDS-associated liver injury are due to multi-ingredient nutritional supplements, and the component responsible for the toxicity is usually unknown or can only be suspected. HDS-induced liver injury presents many clinical and research challenges in diagnosis, identification of the responsible constituents, treatment, and prevention. Also important are improvements in regulatory oversight of nonprescription products to guarantee their constituents and ensure purity and safety. The confident identification of injurious ingredients within HDS will require strategic alignments among clinicians, chemists, and toxicologists. The ultimate goal should be to prohibit or more closely regulate potentially injurious ingredients and thus promote public safety. (Hepatology 2017;65:363-373).
© 2016 by the American Association for the Study of Liver Diseases.
Conflict of interest statement
Figures
References
-
- Navarro VJ, Lucena MI. Hepatotoxicity induced by herbal and dietary supplements. Semin Liver Dis. 2014;34:172–193. - PubMed
-
- Food and Drug Administration. [Accessed February 20, 2016];Dietary Supplements. 2016 Jan 21; Available at http://www.fda.gov/Food/DietarySupplements/default.htm.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical