Hippocampus-associated causal network of structural covariance measuring structural damage progression in temporal lobe epilepsy
- PMID: 27677885
- PMCID: PMC6866709
- DOI: 10.1002/hbm.23415
Hippocampus-associated causal network of structural covariance measuring structural damage progression in temporal lobe epilepsy
Abstract
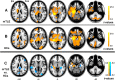
In mesial temporal lobe epilepsy (mTLE), the causal relationship of morphometric alterations between hippocampus and the other regions, that is, how the hippocampal atrophy leads to progressive morphometric alterations in the epileptic network regions remains largely unclear. In this study, a causal network of structural covariance (CaSCN) was proposed to map the causal effects of hippocampal atrophy on the network-based morphometric alterations in mTLE. It was hypothesized that if cross-sectional morphometric MRI data could be attributed temporal information, for example, by sequencing the data according to disease progression information, GCA would be a feasible approach for constructing a CaSCN. Based on a large cohort of mTLE patients (n = 108), the hippocampus-associated CaSCN revealed that the hippocampus and the thalamus were prominent nodes exerting causal effects (i.e., GM reduction) on other regions and that the prefrontal cortex and cerebellum were prominent nodes being subject to causal effects. Intriguingly, compensatory increased gray matter volume in the contralateral temporal region and post cingulate cortex were also detected. The method unraveled richer information for mapping network atrophy in mTLE relative to the traditional methods of stage-specific comparisons and structured covariance network. This study provided new evidence on the network spread mechanism in terms of the causal influence of hippocampal atrophy on progressive brain structural alterations in mTLE. Hum Brain Mapp 38:753-766, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: granger causality analysis; morphometric MRI; network of structural covariance; progression; temporal lobe epilepsy.
© 2016 Wiley Periodicals, Inc.
Figures




References
-
- Alhusaini S, Doherty CP, Scanlon C, Ronan L, Maguire S, Borgulya G, Brennan P, Delanty N, Fitzsimons M, Cavalleri GL (2012): A cross‐sectional MRI study of brain regional atrophy and clinical characteristics of temporal lobe epilepsy with hippocampal sclerosis. Epilepsy Res 99:156–166. - PubMed
-
- Bartolomei F, Wendling F, Bellanger JJ, Regis J, Chauvel P (2001): Neural networks involving the medial temporal structures in temporal lobe epilepsy. Clin Neurophysiol 112:1746–1760. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

